Abi-Rached L, Jobin MJ, Kulkarni S, McWhinnie A, Dalva K, Gragert L, Babrzadeh F, Gharizadeh B, Luo M, Plummer FA, et al. 2011. The shaping of modern human immune systems by multiregional admixture with archaic humans. Science 334(6052):89–94.
Behrensmeyer AK. 2008. Paleoenvironmental context of the Pliocene A.L. 333 “first family” hominin locality, Hadar formation, Ethiopia. GSA Spec Pap. [accessed 2015 Sept 10];446:203–214. http://specialpapers.gsapubs.org/content/446/203.abstract.
Berger LR. 2012. Australopithecus sediba and the earliest origins of the genus Homo. J Anthropol Sci. 90:117–131.
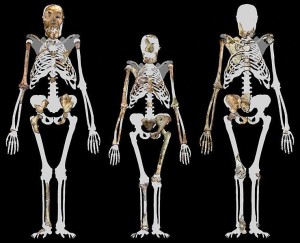
Berger LR, de Ruiter DJ, Schmid SE, Carlson KJ, Dirks PHGM, Kibii JM. 2010. Australopithecus sediba: A new species of Homo-like Australopith from South Africa. Science 328(5975):195–204.
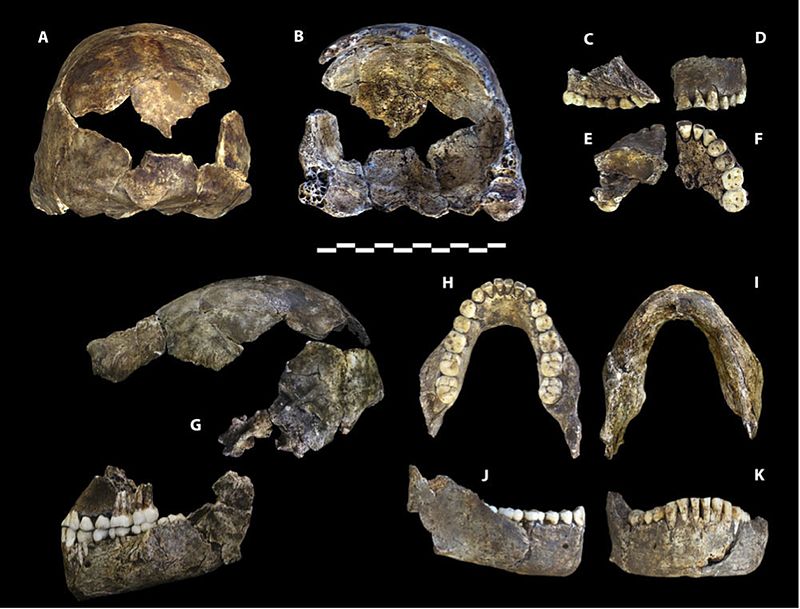
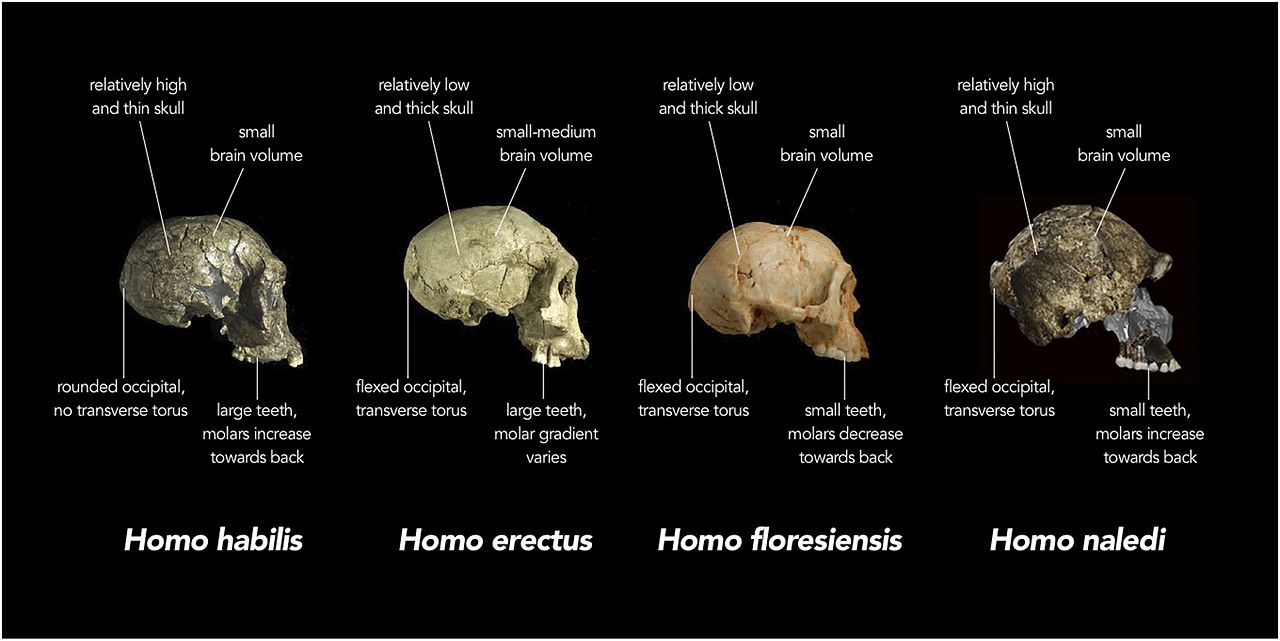
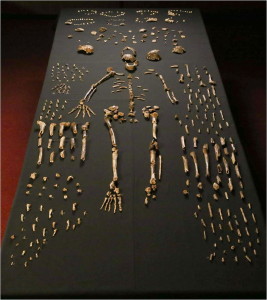
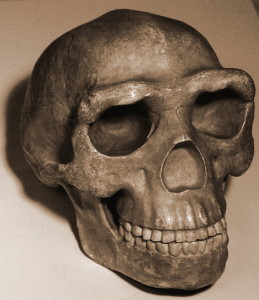
Berger LR, Hawks J, de Ruiter DJ, Churchill SE, Schmid P, Delezene LK, Kivell TL, Garvin HM, Williams SA, DeSilva JM. (2015) Homo naledi, a new species of Homo from the Dinaledi Chamber, South Africa. eLife doi:10.7554/eLife.09560
Blain H. 2012. An environmental tale from Pleistocene Java [MA thesis]. [Leiden, Netherlands]: Leiden University.
Boaz N. 1998. Eco homo: how the human being emerged from the cataclysmic history of the earth. New York (NY): Basic Books.
Bramble DM, Lieberman DE. 2004. Endurance running and the evolution of Homo. Nature 432:345–352.
Bromage TG, McMahon JM, Thackery JF, Kullmar O, Hogg R, Rosenberger AL, Schrenk F, Enlow DH. 2008. Craniofacial architectural constraints and their importance for reconstructing the early Homo skull KNM-ER 1470. J Clinical Pediatr Dent. 33(1):43–54.
Brown P, Sutikna T, Morwood MJ, Soejono RP, Jatmiko, Saptomo EW, Due RA. 2004. A new small-bodied hominin from the Late Paleistocene of Flores, Indonesia. Nature 431:1055–1061.
Bunn HT, Gurtov AN. 2014. Prey mortality profiles indicate that Early Pleistocene Homo at Olduvai was an ambush predator. Quatern Int. 322–323:44–53.
Callaway E. 2013. Hominin DNA baffles experts. Nature 504:16–17.
Campbell BG. 1998. Human evolution: an introduction to man’s adaptations. 4th ed. New York (NY): Aldine de Gruyter.
Carlson KJ, Stout D, Jashashvili T, de Ruiter DJ, Tafforeau P, Carlson K, Berger LR. 2011. The endocast of MH1, Australopithecus sediba. Science 333(6048):1402–1407.
Cartmill M, Smith FH. 2009. The Human Lineage. New York: Wiley-Blackwell.
Chang CH, Kaifu Y, Takai M, Kono RT, Grün R, Matsu’ura S, Kinsley L, Lin LK. 2015. The first archaic Homo from Taiwan. Nat Commun. 6:6037. doi:10.1038/ncomms7037.
Clement A, Hillson S. 2013 Oct 24. ‘Do larger molars and robust jaws in early hominins represent dietary adaptation?’ a new study in tooth wear. UCL Archaeol Int.; [accessed 2015 Oct 3]. doi:10.5334/ai.1605.
Copeland SR, Sponheimer M, de Ruiter DJ, Lee-Thorp JA, Codron D, le Roux PJ, Grimes V, Richards MP. 2011. Strontium isotope evidence for landscape use by early hominins. Nature 474:76–78.
Curnoe D, Ji XP, Herries AIR, Kanning B, Taçon PSC, Zhende B, Fink D, Yunsheng Z, Hellstrom J, Yun L, et al. 2012. Human remains from the Pleistocene-Holocene transition of Southwest China suggest a complex evolutionary history for East Asians. PLoS One 7(3):e31918.
Davis, OK. 2009. Other dating methods.Tucson (AZ): University of Arizona; [accessed 2015 Sept 16].http://www.geo.arizona.edu/palynology/geos462/11datingmeth.html.
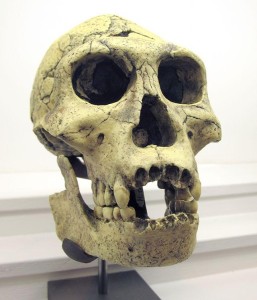
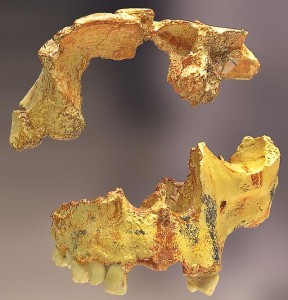
de Lumley H, Nioradze M, Barsky D, Cauche D, Celiberti V, Nioradze G, Zvania D, Lordkipanidze D. 2005. The pre-Oldowayen lithic industry from the beginning of the Lower Pleistocene at the Dmanisi site in Georgia. Anthropologie 109:1–182.
de Lumley M, Lordkipanidze D. 2006. L’home de dmanissi (Homo georgicus), il y a 18100000 ans. C R Palevol. 5:273–281.
de Ruiter DJ, DeWitt TJ, Carlson KB, Brophy JK, Schroeder L, Ackermann RR, Churchill SE, Berger LR. 2013. Mandibular remains support taxonomic validity of Australopithecus sediba. Science 340(6129):1232997.
DeSilva JM, Holt KG, Churchill SE, Carlson KJ, Walker CS, Zipfel B, Berger LR. 2013.The lower limb and mechanics of walking in Australopithecus sediba. Science 340(6129):1232999.
DiMaggio EN, Campisano CJ, Rowan J, Dupont-Nivet G, Deino AL, Bibi F, Lewis ME, Souron A, Werdelin L, Reed KE, et al. 2015. Late Pliocene fossiliferous sedimentary record and the environmental context of early Homo from Afar, Ethiopia. Science 347(6228):1355–1359. doi:10.1126/science.aaa1415.
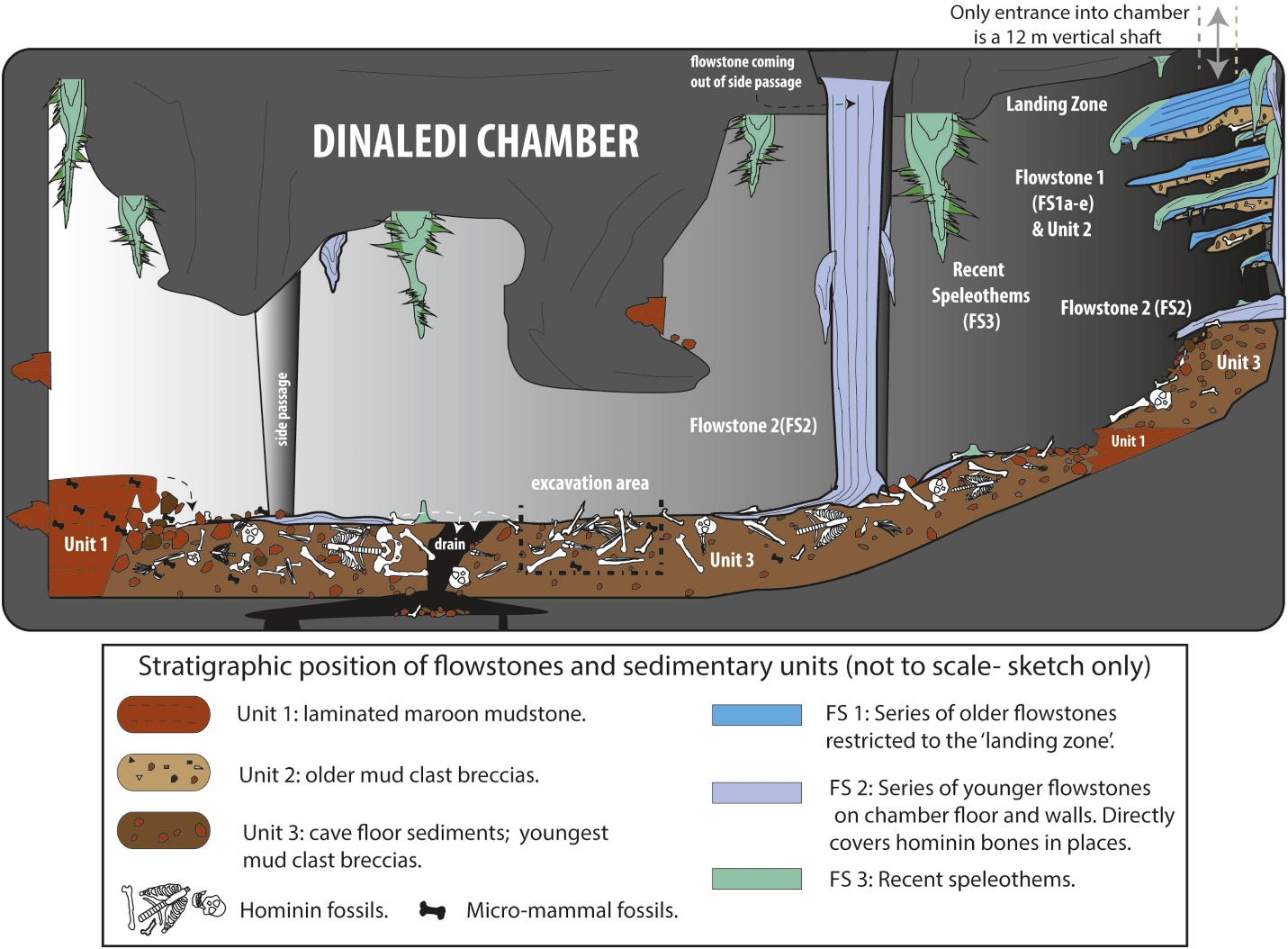
Dirks P HGM, Berger LR, Roberts EM, Kramers JD, Hawks J, Randolph-Quinney PS, Elliott M, Musiba CM, Churchill SE, de Ruiter DJ, et al. (2015) Geological and taphonomic context for the new hominin species Homo naledi from the Dinaledi Chamber, South Africa. DOI: doi:10.7554/eLife.09561.
Duke L. 1998 Dec 10. Full Australopithecus fossil found in South Africa. Washington Post. Sect. A:1; [accessed 2015 Sept 10]. http://www.washingtonpost.com/wp-srv/national/daily/dec98/safrica10.htm.
Fagan BM. 2000. Chronological methods 11 – Paleomagnetic and archaeomagnetic dating. Santa Barbara (CA): Regents of the University of California; [accessed 2015 Aug 30]. http://archserve.id.ucsb.edu/courses/anth/fagan/anth3/Courseware/Chronology/11_Paleomag_Archaeomag.html.
Falk D. 2009. Finding our tongues: Mothers, infants and the origins of language. New York, (NY): Basic Books.
Falk D, Hildebolt C, Smith K, Morwood MJ, Sutikna T, Brown P, Jatmiko, Wavhu Saptomo E, Brunsden B, Prior F. 2005. The brain of LB1, Homo floresiensis. Science 308(5719):242-245.
Gibbons A. 2009. A new kind of ancestor: Ardipithecus unveiled. Science 326:36-40.
Green DJ, Gordon AD, Richmond BG. 2007. Limb-size proportions in Australopithecus afarensis and Australopithecus africanus. J Hum Evol. 52(2):187–200.
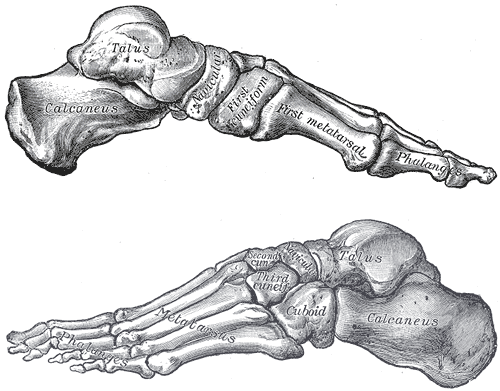
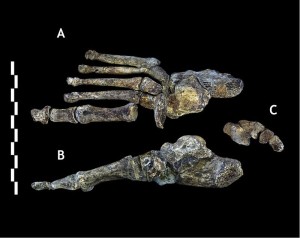
Harcourt-Smith WEH, Throckmorton Z, Congdon KA, Zipfel B, Deane AS, Drapeau MSM, Churchill SE, Berger LR, DeSilva JM (2015). The foot of Homo naledi. Nature Communications 6:8432. doi:10.1038/ncomms9432.
Harmund S, Lewis JE, Feibel CS, Lepre CJ, Prat S, Lenoble A, Boës X, Quinn RL. 2015. 3.3-million-year-old stone tools from Lemokwi 3, West Turkana, Kenya. Nature 521:310–315. doi:10.1038/nature14464.
Hawks J, de Ruiter DJ, Berger LR. 2015. Comment on “Early Homo at 2.8 Ma from Ledi-Geraru, Afar, Ethiopia.” Science 348(6241):1326. doi:10.1126/science.aab0591.
Henke W, Tattersall I, editors. 2006. Handbook of paleoanthropology. New York (NY): Springer; [accessed 2015 Aug 15]. http://www.evolbiol.ru/large_files/handbook_paleoanthropology.pdf.
Henry AG. 2011. Microfossils in calculus demonstrate consumption of plants and cooked foods in Neanderthal diets. Proc Natl Acad Sci USA 108(2):486–491.
Henry AG, Ungar PS, Passey BH, Sponheimer M, Rossou L, Bamford M, Sandberg P, de Ruiter DJ, Berger LR. 2012. The diet of Australopithecus sediba. Nature 487:90–93.
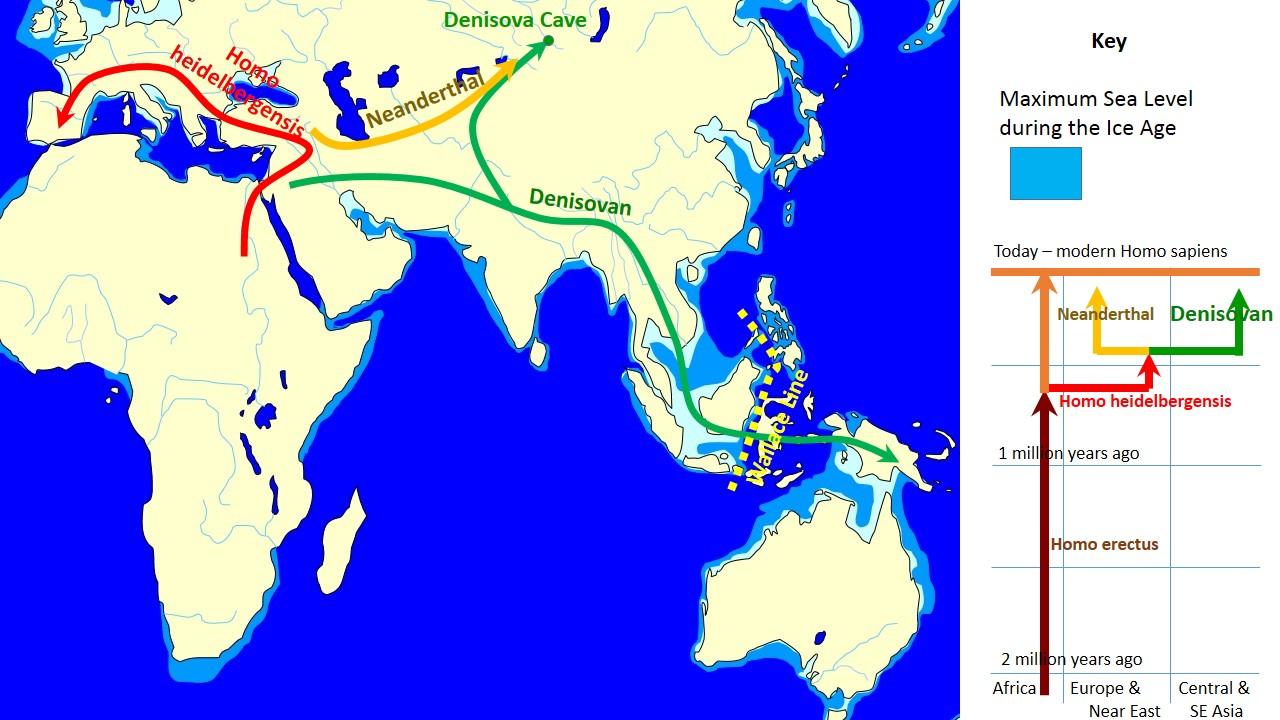
Huerta-Sánchez E, Jin X, Asan, Bianba Z, Peter BM, Vinckenbosch N, Liang Y, Yi X, He M, Somel M, et al. 2014. Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA. Nature 512:194–197.
Irish JD, Guatelli-Steinberg D, Legge SS, de Ruiter DJ, Berger LR. 2013. Dental morphology and the phylogenetic “place” of Australopithecus sediba. Science 340(6129):1233062.
Ji X, Curnoe D, Bao Z, Herries AIR, Fink D, Zhu Y, Hellstrom J, Luo Y, Taçon PSC. 2013. Further ecological excavations at the Maludong hominin site, Yunnan Province, Southwest China. Chinese Sci Bull. 58(35):4472–4485.
Jungers WL, Larson SG, Harcourt-Smith W, Morwood MJ, Sutikna T, Due AR, Djubiantono T. 2008. Descriptions of the lower limb skeleton of Homo floresiensis. J Hum Evol. 57(5):538–554.
Kibii JM, Churchill SE, Schmid P, Carlson KJ, Reed MD, de Ruiter DJ, Berger LR. 2011. A partial pelvis of Australopithecus sediba. Science 333(6048):1407–1411.
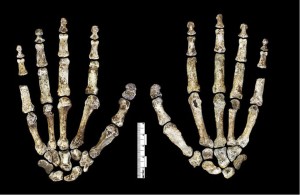
Kivell TL, Deane AS, Tocheri MW, Orr CM, Schmid P, Hawks J, Berger LR, Churchill SE. (2015) The hand of Homo naledi. Nature Communications 6:8431. doi:10.1038/ncomms9431.
Kivell TL, Kibii JM, Churchill SE, Schmid P, Berger LR. 2011. Australopithecus sediba hand demonstrates mosaic evolution of locomotor and manipulative abilities. Science 333(6048):1411–1417.
Lehmkuhl F, Klinge M, Stauch G. 2011. The extent and timing of late Pleistocene glaciations in the Altai and neighboring mountain systems. Dev Quatern Sci. 15:967–979.
Le Gros Clark WE. 1959. The antecedents of man. New York (NY): Harper and Row.
Lovejoy CO. 1981. The origins of man. Science 211:341-348.
Lovejoy CO. 1988. Evolution of human walking. Scientific American, November 1988: 118-125.
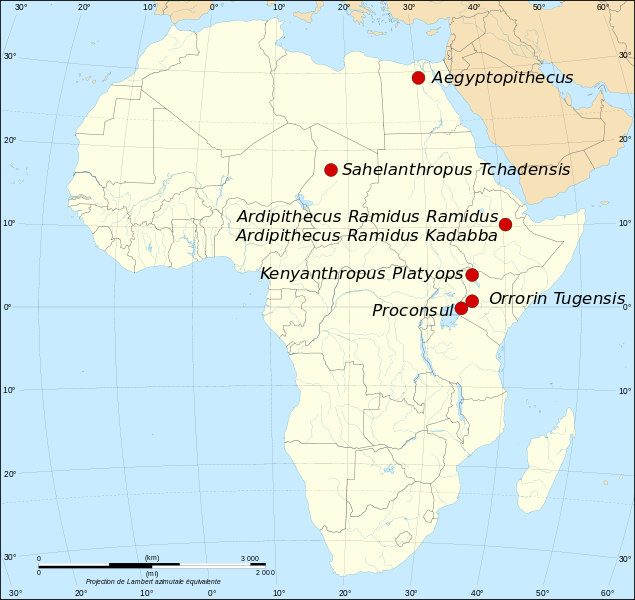
Lovejoy CO, Latimer B, Suwa G, Asfaw B, White TD. 2009. Combining prehension and propulsion: the foot of Ardipithecus ramidus. Science 326(5949):72e1–72e8.
Marshall M. 2013 Nov 19. Mystery human species emerges from Denisovan genome. New Sci. [accessed 2015 Sept 29]. http://www.newscientist.com/article/dn24603-mystery-human-species-emerges-from-denisovan-genome.html.
Martínez I, Rosa M, Arsuaga JL, Jarabo P, Quam R, Lorenzo C, Gracia A, Carretero JM, Bermúdez de Castro JM. 2004. Auditory capacities in Middle Pleistocene humans from the Sierra de Atapuerca in Spain. Proc Natl Acad Sci USA 101:9976–9981.
McHenry, H. 2015. Australopithecus. Encyclopædia Britannica Online; [accessed 2015 Sept 18]. http://www.britannica.com/topic/Australopithecus.
McHenry HM, Berger LR. 1998. Body proportions of Australopithecus afarensis and A. africanus and the origin of the genus Homo. J Hum Evol. 35(1):1–22.
Morwood MJ, Brown P, Jatmiko, Sutikna T, Saptomo EW, Westaway KE, Due RA, Roberts RG, Maeda T, Wasisto S, et al. 2005. Further evidence for small-bodied hominins from the Late Pleistocene of Flores, Indonesia. Nature 437:1012–1017.
Morwood MJ, O’Sullivan P, Aziz F, Raza A. 1998. Fission track age of stone tools and fossils on the east Indonesian island of Flores. Nature 392:173–176.
Napier JR, Napier PH. 1967. A handbook of the living primates. London: Academic Press.
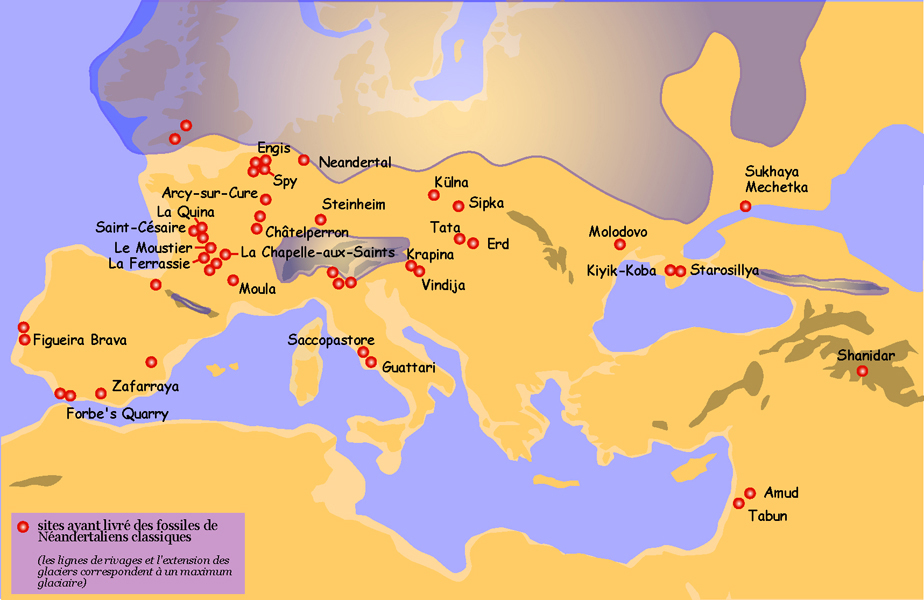
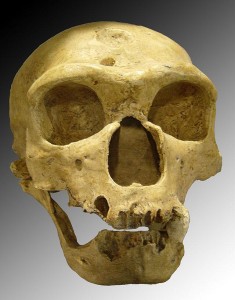
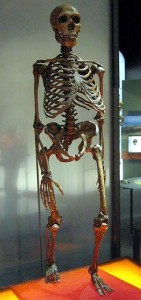
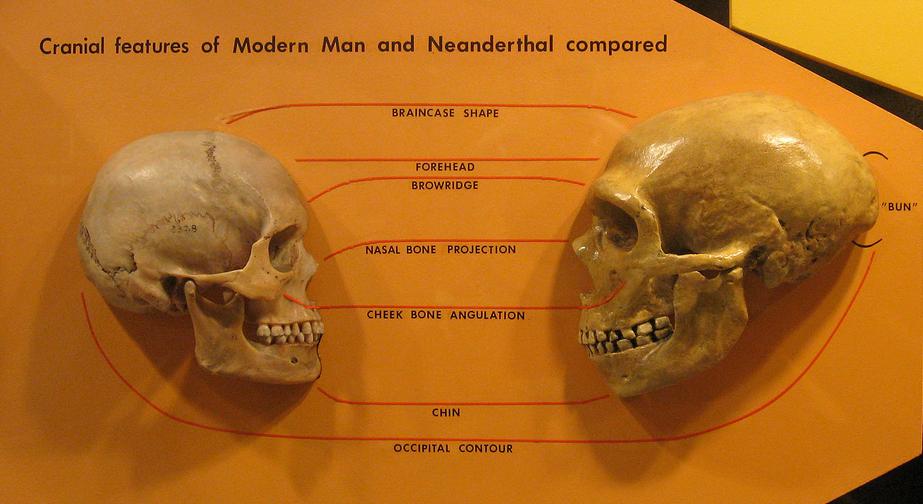
Pääbo S. 2014. Neanderthal man: in search of lost genomes. New York (NY): Basic Books.
Pickering R, Dirks PHGM, Jinnah Z, de Ruiter DJ, Churchill SE, Herries AIR, Woodhead JD, Hellstrom JC, Berger LR. 2011. Australopithecus sediba at 1.977 Ma and implications for the origins of the genus Homo. Science 333(6048):1421–1423.
Prüfer K, Racimo F, Patterson N, Jay F, Sankararaman S, Sawyer S, Heinze A, Renaud G, Sudmant PH, De Filippo C, et al. 2013. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505:43–49.
Randolph-Quinney PS. A new star rising: Biology and mortuary behavior of Homo naledi. South African Journal of Science 1111 (9/10): 2-5.
Reich D, Green RE, Kircher M, Krause J, Patterson N, Durand EY, Viola B, Briggs AW, Stenzel U. 2010. Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature 468:1053–1060.
Schmid P, Churchill SE, Nalla S, Weissen E, Carlson KJ, de Ruiter DJ, Berger LR. 2013. Mosaic morphology in the thorax of Australopithecus sediba. Science 340(6129):1234598.
Smithsonian Institution. 2015. Homo heidelbergensis. What does it mean to be human?; [accessed 2015 Sept 22]. http://humanorigins.si.edu/evidence/human-fossils/species/homo-heidelbergensis.
Smithsonian Institution. 2015. Ardipithecus ramidus. What does it mean to be human?; [accessed 2016 June 14]. http://humanorigins.si.edu/evidence/human-fossils/species/ardipithecus-ramidus.
Stringer C, Andrews P. 2005. The complete world of human evolution. London (England): Thames and Hudson.
Tattersall I. 2009. The fossil trail: How we know what we think we know about human evolution, 2nd ed. New York: Oxford University Press.
Thacheray JF (2015) Estimating the age of Homo naledi. South African Journal of Science 111(11/12):3–4. doi:10.17159/sajs.2015/a0124.
Villmoare B, Kimbel WH, Seyoum C, Campisano CJ, DiMaggio A, Rowan J, Braun DR, Arrowsmith JR, Reed KE. 2015. Early Homo at 2.8 Ma from Ledi-Geraru, Afar, Ethiopia. Science 347(6228):1352–1355. doi:10.1126/science.aaa1343.
Wagner GA. 2006. Chronometric methods in paleoanthropology. In: Henke W, Tattersall I, editors. Handbook of paleoanthropology. New York (NY): Springer. p. 311–337.
Wayman E. 2012. Sahelanthropus tchadensis: Ten years after the discovery. Smithsonian.com; [accessed 2015 Sept 7]. http://www.smithsonianmag.com/science-nature/sahelanthropus-tchadensis-ten-years-after-the-disocvery-2449553/.
Wikipedia contributors. 2015a. Cueva de La Pasiega. Wikipedia; [accessed 2015 Sept 29]. https://en.wikipedia.org/wiki/Cueva_de_La_Pasiega.
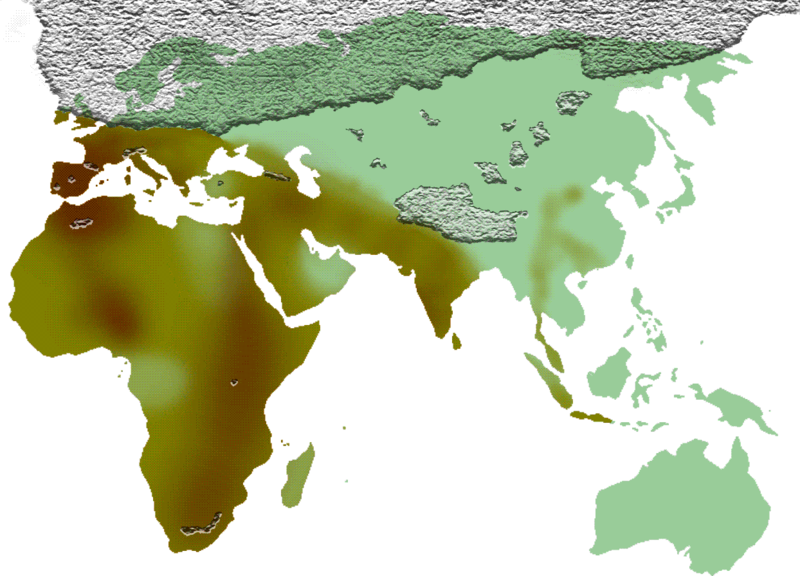
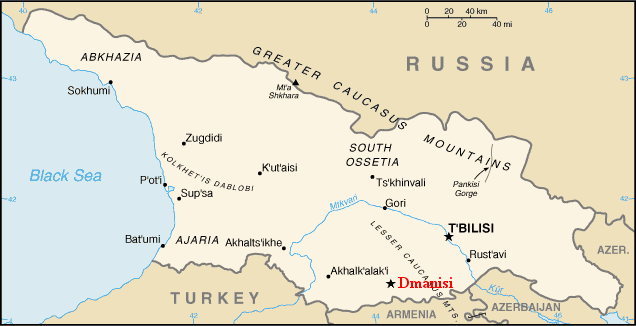
Wikipedia contributors. 2015b. Dmanisi. Wikipedia; [accessed 2015 Sept 20]. https://en.wikipedia.org/wiki/Dmanisi.
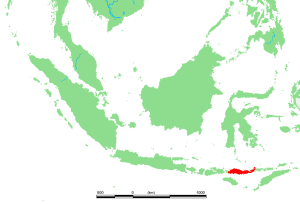
Wikipedia contributors. 2015c. Flores. Wikipedia; [accessed 2015 Sept 22]. https://en.wikipedia.org/wiki/Flores.
Wikipedia contributors. 2015d. Homo floresiensis. Wikipedia; [accessed 2015 Sept 22]. https://en.wikipedia.org/wiki/Homo_floresiensis.
Wikipedia contributors. 2015e. Hoxnian stage. Wikipedia; [accessed 2015 Sept 22]. https://en.wikipedia.org/wiki/Hoxnian_Stage.
Wikipedia contributors. 2015f. Malapa fossil site, cradle of humankind. Wikipedia; [accessed 2015 Sept 11]. https://en.wikipedia.org/wiki/Malapa_Fossil_Site,_Cradle_of_Humankind.
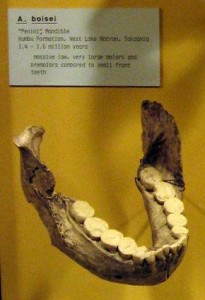
Wikipedia contributors. 2015g. Paranthropus boisei. Wikipedia; [accessed 2015 Sept 11]. https://en.wikipedia.org/wiki/Paranthropus_boisei.
Wikipedia contributors. 2015h. Robert Broom. Wikipedia; [accessed 2015 Sept 10]. https://en.wikipedia.org/wiki/Robert_Broom.
Wikipedia contributors. 2015i. Surface exposure dating. Wikipedia; [accessed 2015 Aug 12]. http://en.wikipedia.org/wiki/Surface_exposure_dating
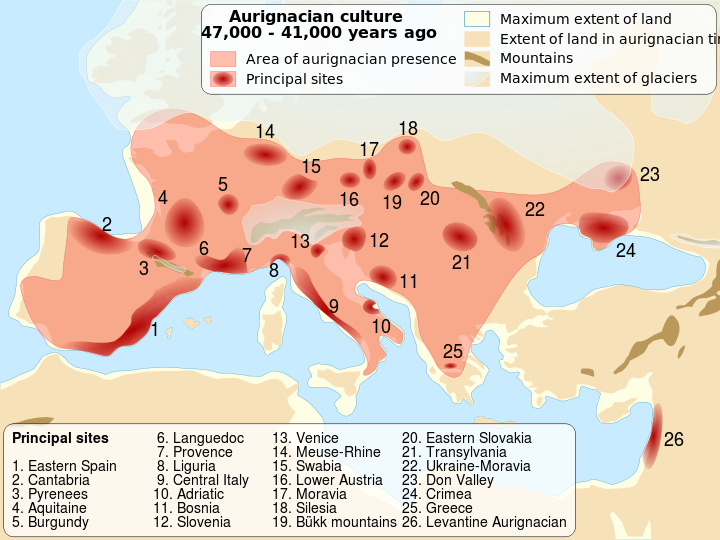
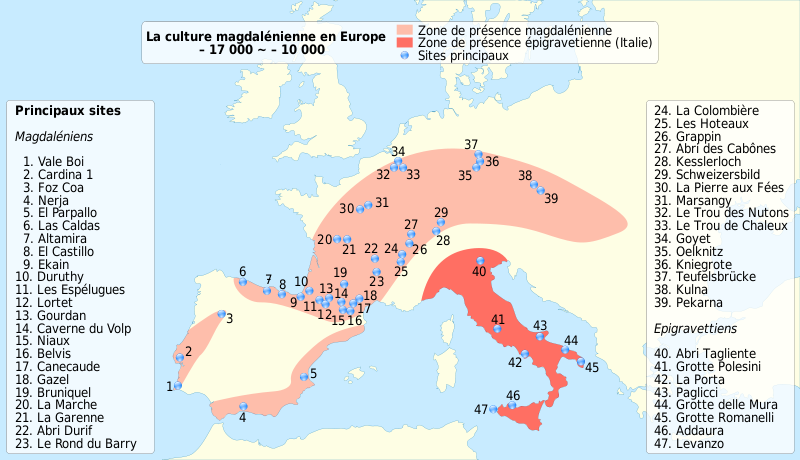
Wikipedia contributors. 2015j. Upper Paleolithic. Wikipedia; [accessed 2015 Sept 29]. https://en.wikipedia.org/wiki/Upper_Paleolithic.
Williams SA, Ostrofsky KR, Frater N, Churchill SE, Schmid P, Berger LR. 2013. The vertebral column of Australopithecus sediba. Science 340(6129):1232996.
Zipfel B, DeSilva JM, Kidd RS, Carlson KJ, Churchill SE, Berger LR. 2011. The foot and ankle of Australopithecus sediba. Science 333(6048):1417–1420.