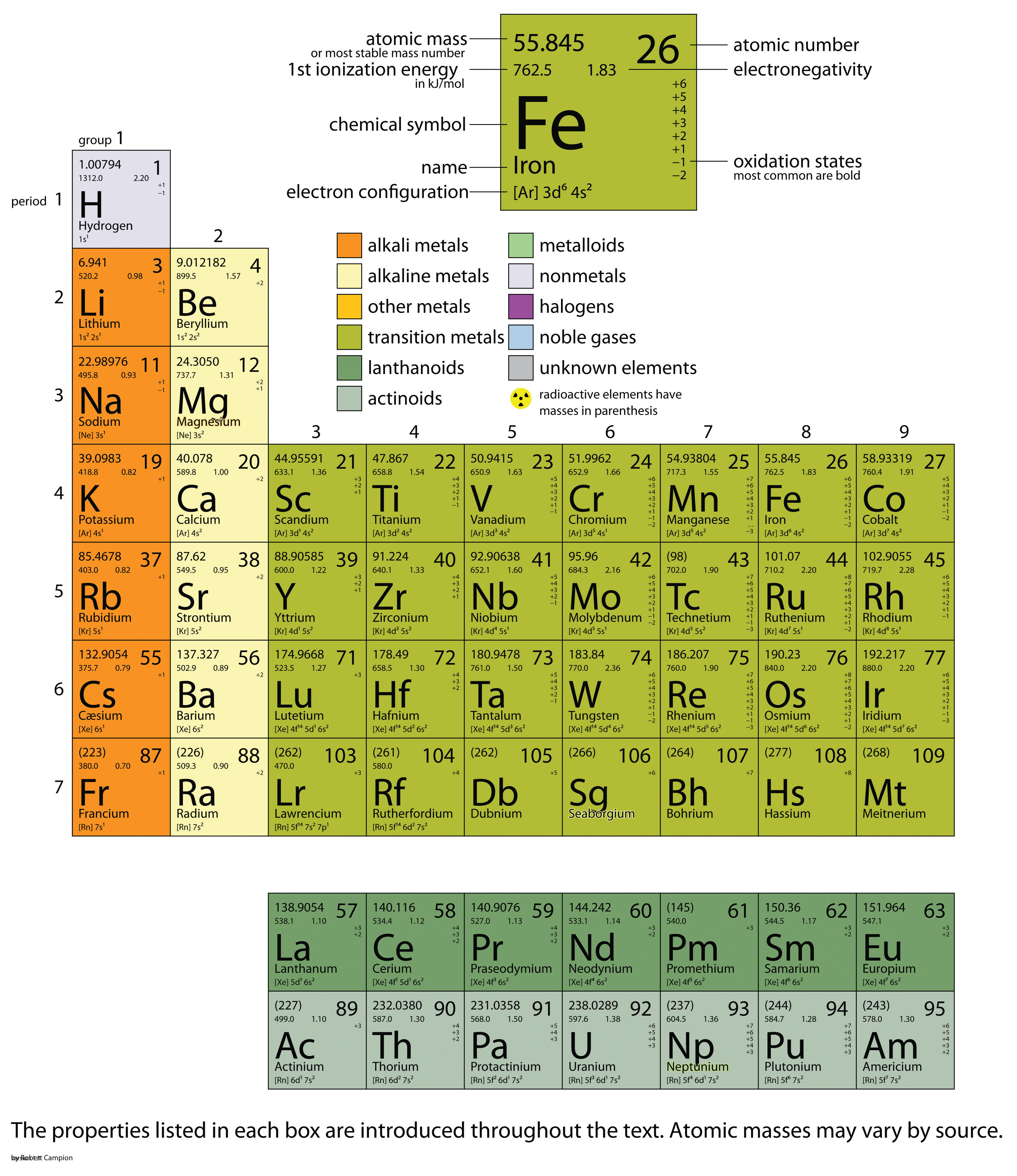
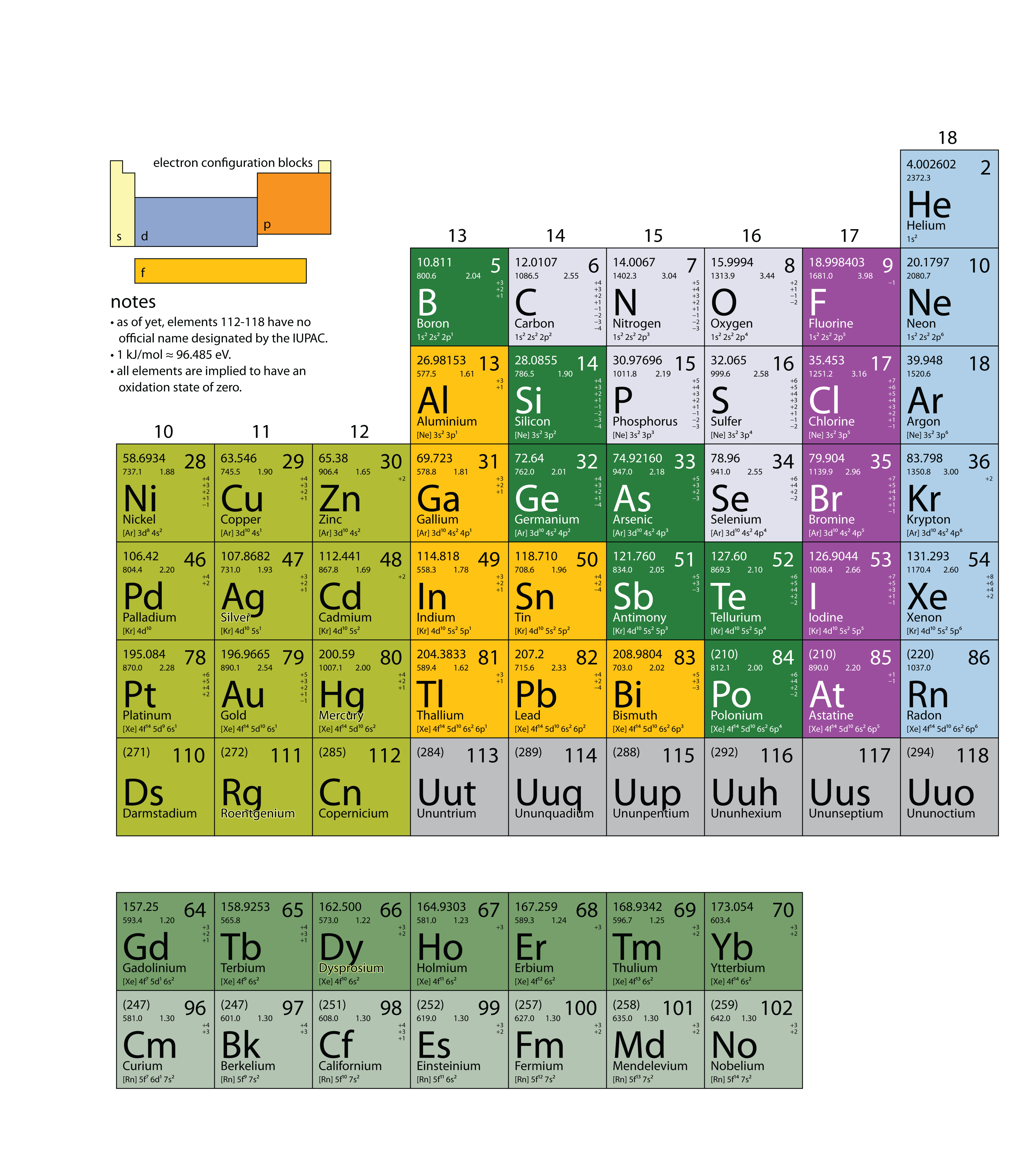
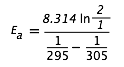| Term | Definition | Section of Book |
| abbreviated electron configuration | An electron configuration that uses one of the noble gases to represent the core of electrons up to that element | Organization of Electrons in Atoms |
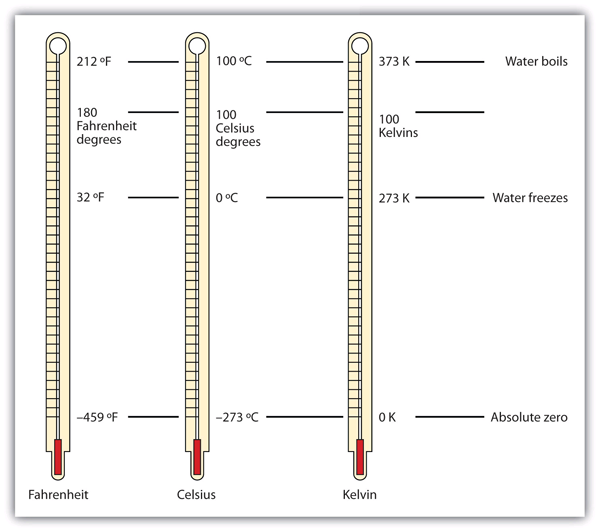
| absolute zero | The minimum possible temperature, labeled 0 K (zero kelvins) | Other Units: Temperature and Density |
| acid | An ionic compound of the H+ cation dissolved in water | Acids |
| acid | A compound that increases the amount of H+ ions in an aqueous solution | Neutralization Reactions |
| acid dissociation constant (Ka) | The equilibrium constant for the dissociation of a weak acid into ions | Some Special Types of Equilibria |
| acid salt | An ionic compound whose aqueous solution is slightly acidic | Strong and Weak Acids and Bases and Their Salts |
| activated complex | See transition state | Reaction Mechanisms |
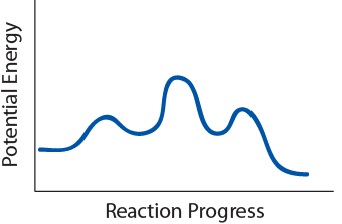
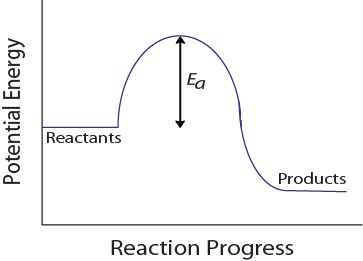
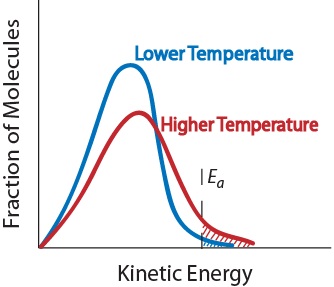
| activation energy (Ea) | The minimum amount of kinetic energy molecules must possess for an effective collision to occur | Factors that Affect the Rate of Reactions |
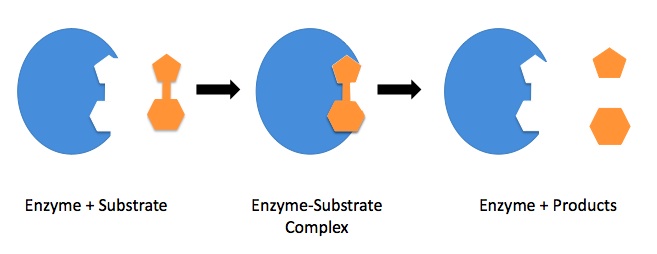
| active site | Area of enzymatic action where substrate molecules react | Catalysis |
| activity series | A list of elements that will replace elements below them in single-replacement reactions | Types of Chemical Reactions: Single- and Double-Displacement Reactions |
| actual yield | The amount that is actually produced in a chemical reaction | Yields |
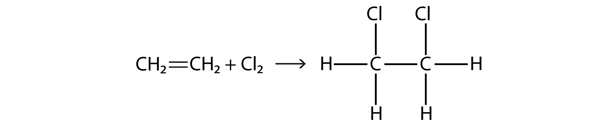
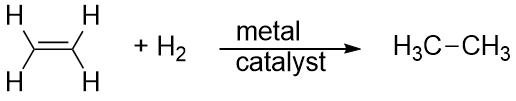
| addition reaction | A reaction where atoms are added across a double or triple bond | Hydrocarbons |
| adhesion | The tendency of a substance to interact with other substances because of intermolecular forces | Properties of Liquids |
| adsorb | Bind to the surface of another substance | Catalysis |
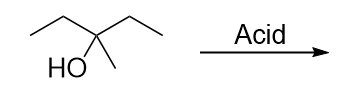
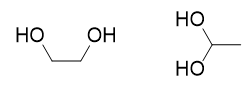
| alcohol | An organic compound that contains an OH functional group | Alkyl halides and alcohols |
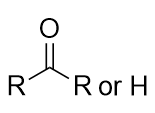
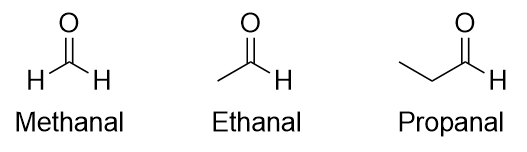
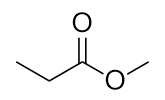
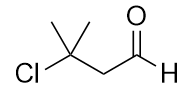
| aldehyde | A compound that has a carbonyl functional group at the end of a chain of C atoms | Other Oxygen-Containing Functional Groups |
| aliphatic hydrocarbons | A hydrocarbon based on chains of C atoms | Hydrocarbons |
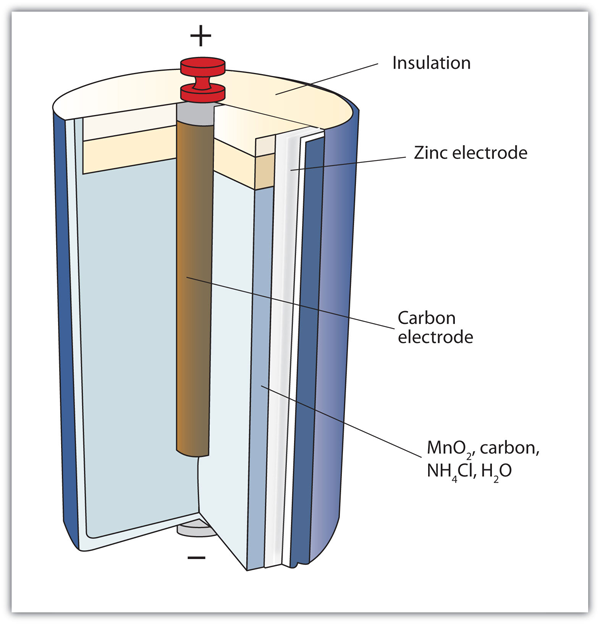
| alkaline battery | A type of dry cell that contains an alkaline (i.e., basic) moist paste, rather than an acidic paste | Applications of Redox Reactions: Voltaic Cells |
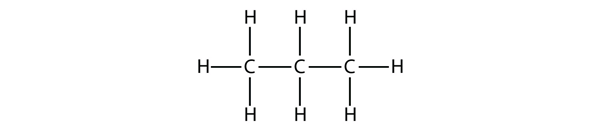
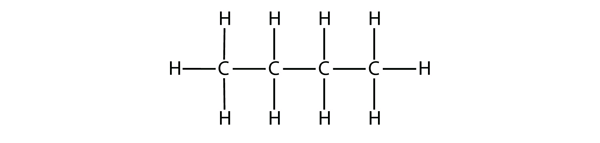
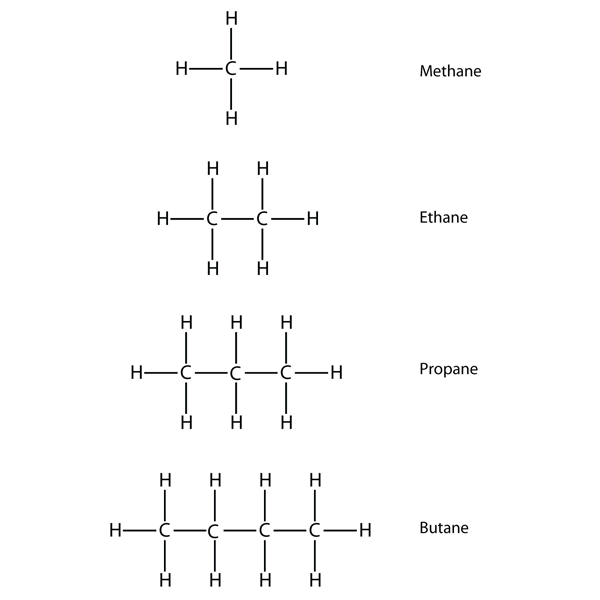
| alkane | An aliphatic hydrocarbon with only single covalent bonds | Hydrocarbons |
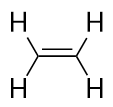
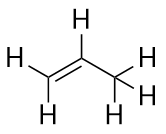
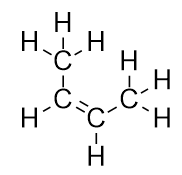
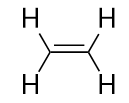
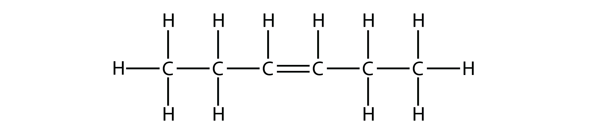
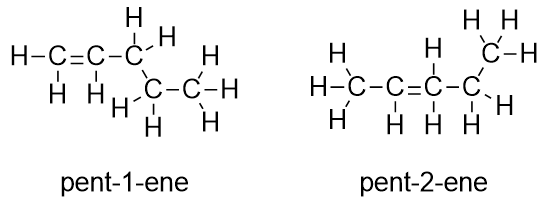
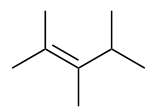
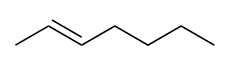
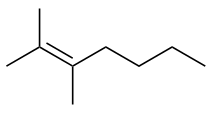
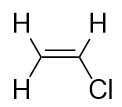
| alkene | An aliphatic hydrocarbon that contains a C–C double bond | Hydrocarbons |
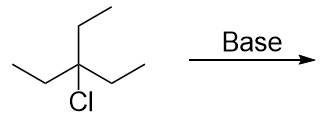
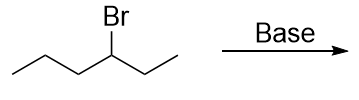
| alkyl halide | An organic compound that contains a halogen atom | Alkyl halides and alcohols |
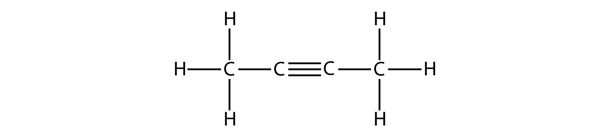
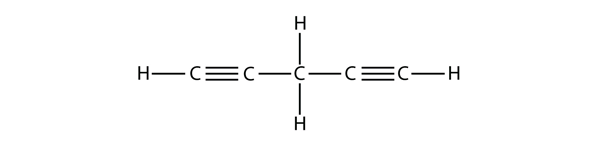
| alkyne | An aliphatic hydrocarbon that contains a C–C triple bond | Hydrocarbons |
| alpha particle | A type of radioactive emission equivalent to a helium nucleus | Radioactivity |
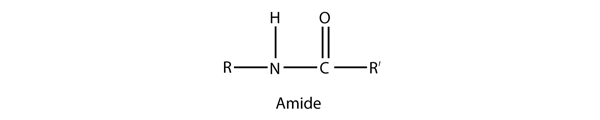
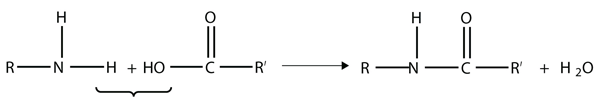
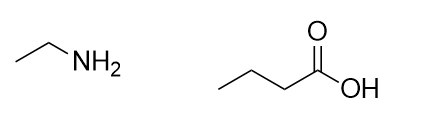
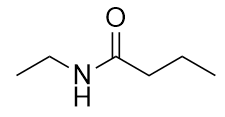
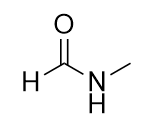
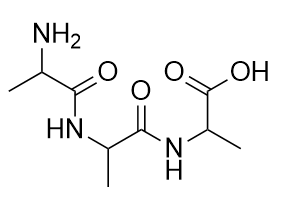
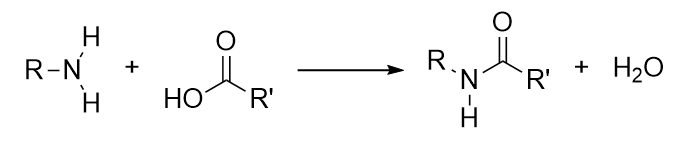
| amide group | A functional group that is the combination of the amine and carbonyl functional groups | Other Functional Groups |
| amide bond | The bond between the N atom and the C atom in an amide. | Other Functional Groups |
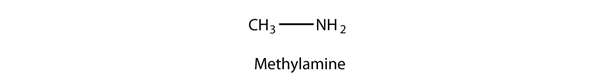
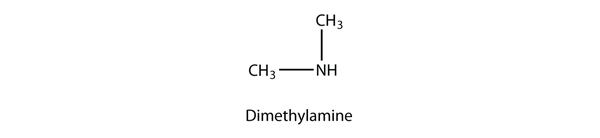
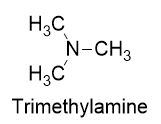
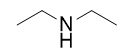
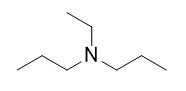
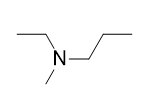
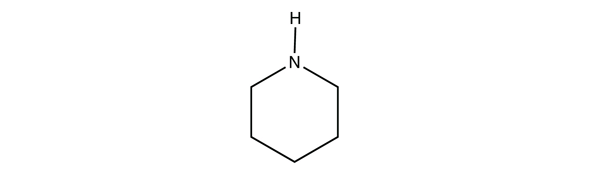
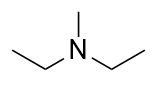
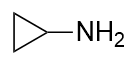
| amine | An organic derivative of ammonia | Other Functional Groups |
| amorphous solid | A solid with no long-term structure or repetition | Solids |
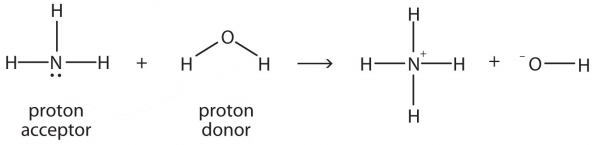
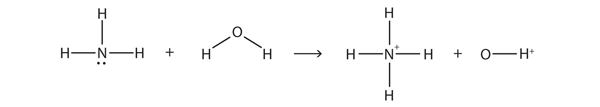
| amphiprotic | A substance that can act as a proton donor or a proton acceptor | Brønsted-Lowry Acids and Bases |
| analyte | The reagent of unknown concentration | Acid-Base Titrations |
| angular momentum quantum number (ℓ) | An index that affects the energy and the spatial distribution of an electron in an atom | Quantum Numbers for Electrons |
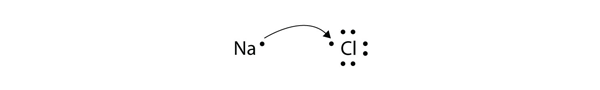
| anion | A species with an overall negative charge | Ions and Ionic Compounds |
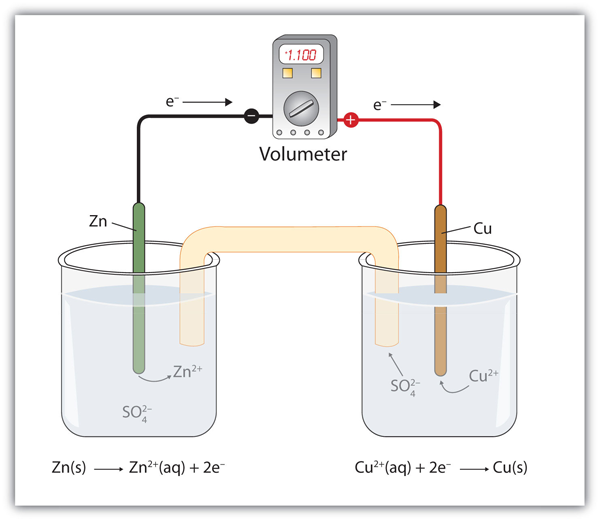
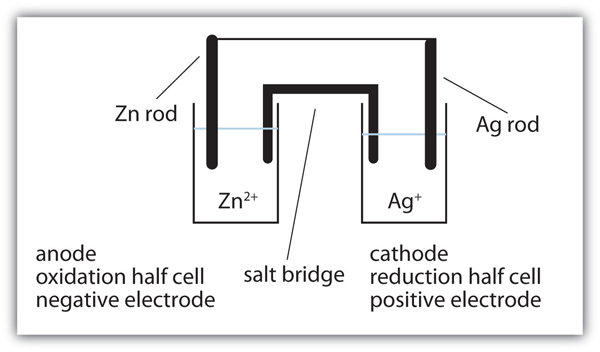
| anode | The half cell that contains the oxidation reaction | Applications of Redox Reactions: Voltaic Cells |
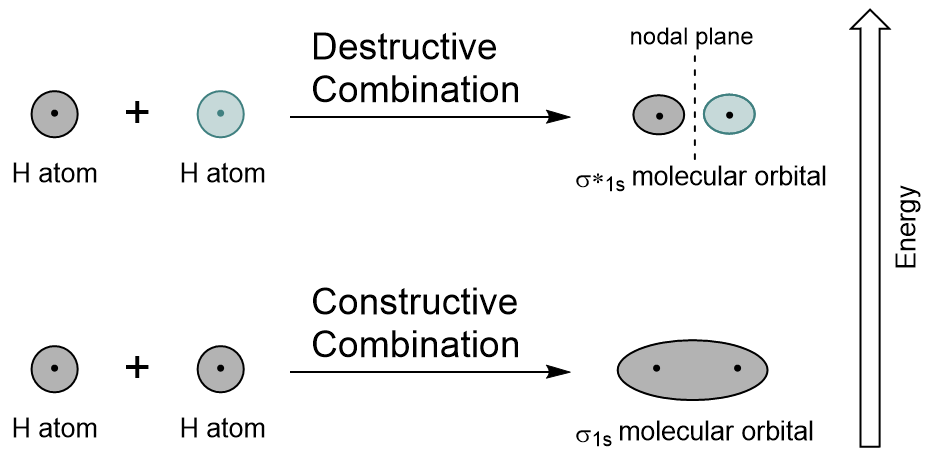
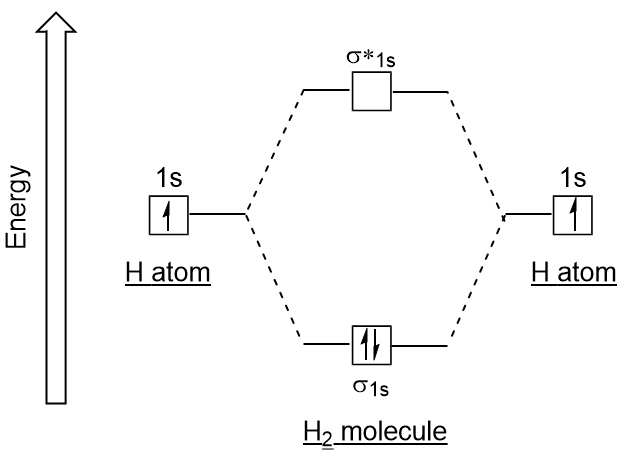
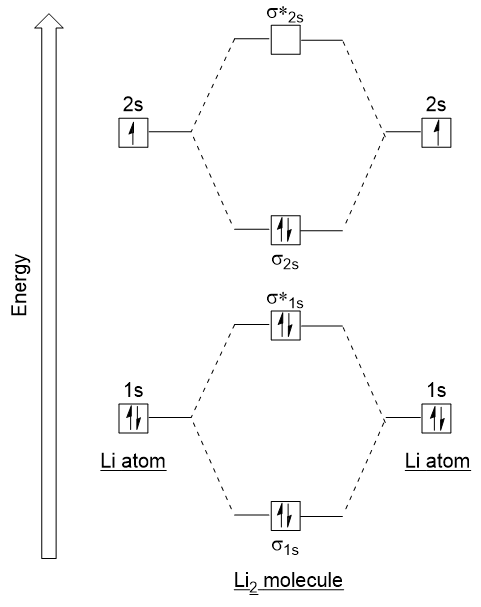
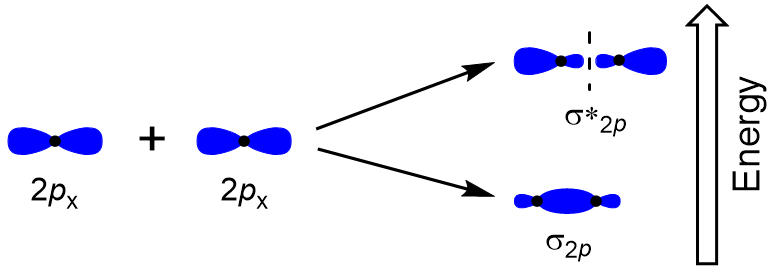
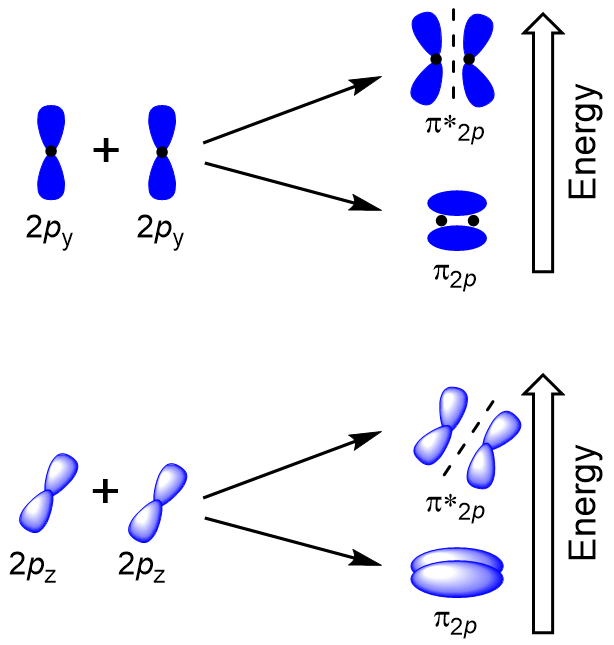
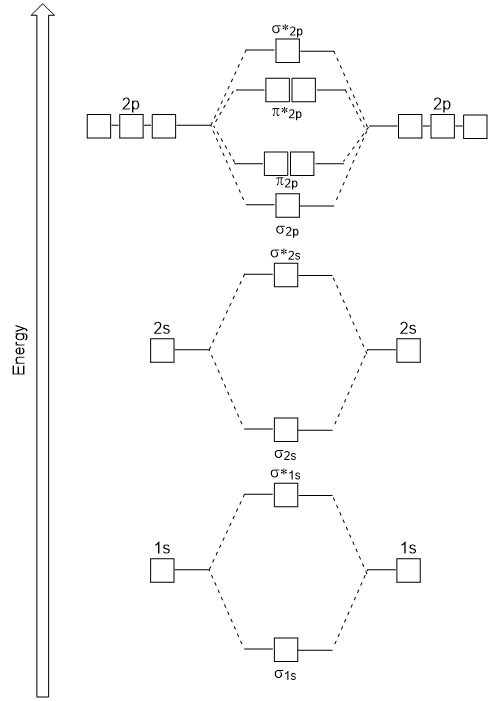
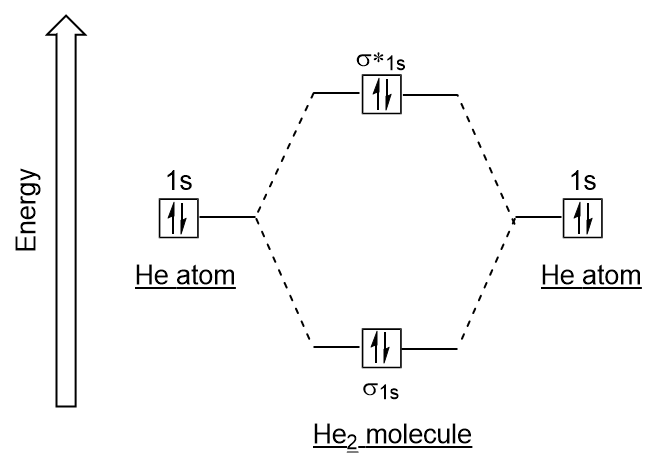
| antibonding molecular orbital | A higher energy molecular orbital generated by destructive combination of atomic orbitals | Molecular Orbitals |
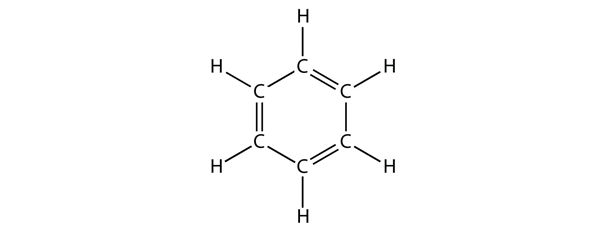
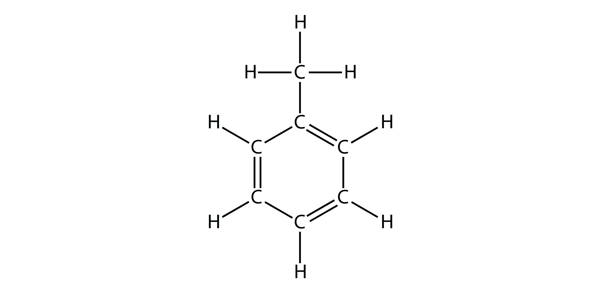
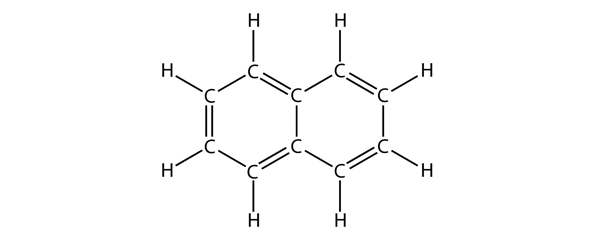
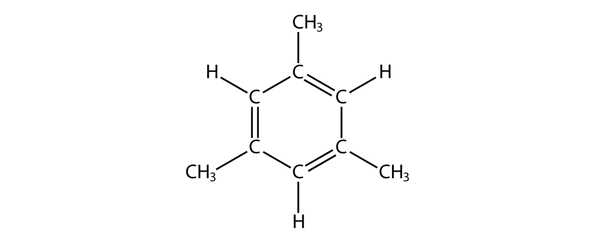
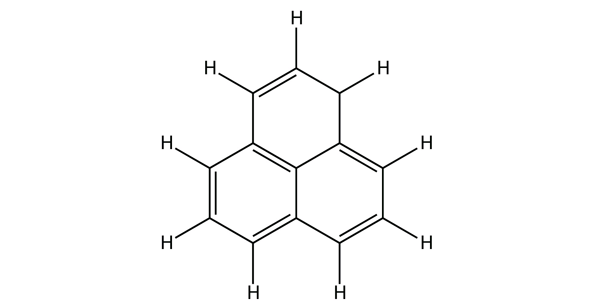
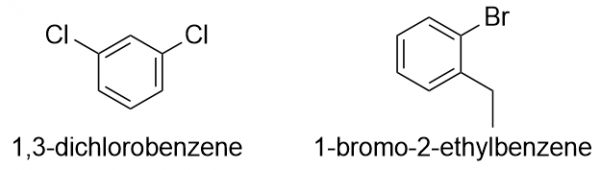
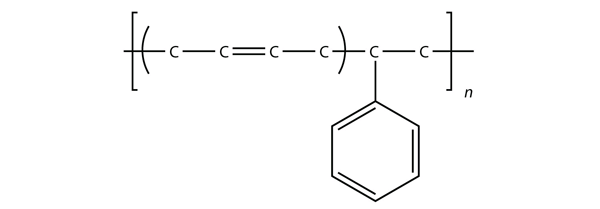
| aromatic hydrocarbons | Flat ring systems, which contain continuously overlapping p orbitals, such as benzene | Hydrocarbons |
| Arrhenius acid | A compound that increases the hydrogen ion concentration in aqueous solution | Arrhenius Acids and Bases |
| Arrhenius base | A compound that increases the hydroxide ion concentration in aqueous solution | Arrhenius Acids and Bases |
| atmosphere (atm) | A unit of pressure equal to the average atmospheric pressure at sea level; defined as exactly 760 mmHg | Pressure |
| atom | The smallest piece of an element that maintains the identity of that element | Atomic Theory |
| atomic mass | The sum of the number of protons and neutrons in a nucleus | Atomic Theory |
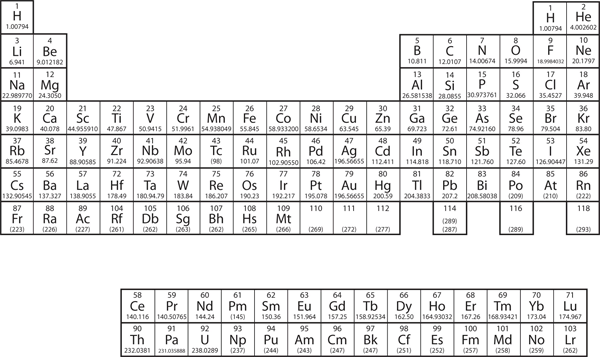
| atomic mass | The weighted average of the masses of the isotopes that compose an element | Masses of Atoms and Molecules |
| atomic mass unit | One-twelfth of the mass of a carbon-12 atom | Masses of Atoms and Molecules |
| atomic number | The number of protons in an atom | Atomic Theory |
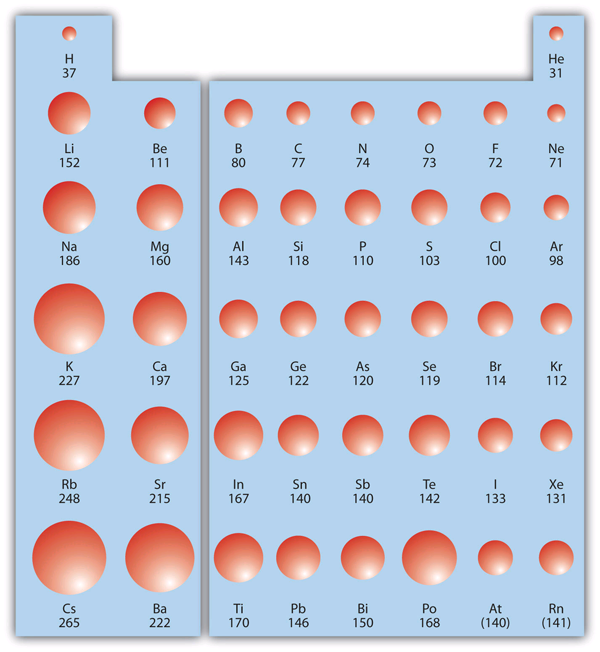
| atomic radius | An indication of the size of an atom | Periodic Trends |
| atomic symbol | A one- or two-letter representation of the name of an element | Atomic Theory |
| atomic theory | The concept that atoms play a fundamental role in chemistry | Atomic Theory |
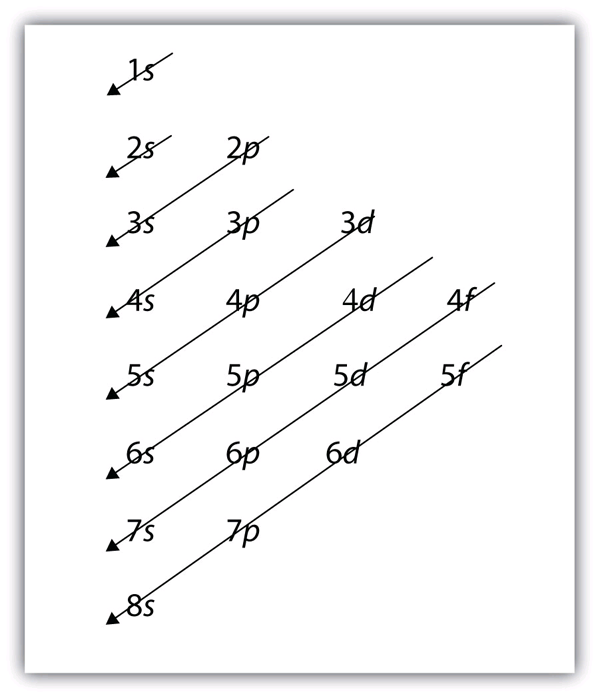
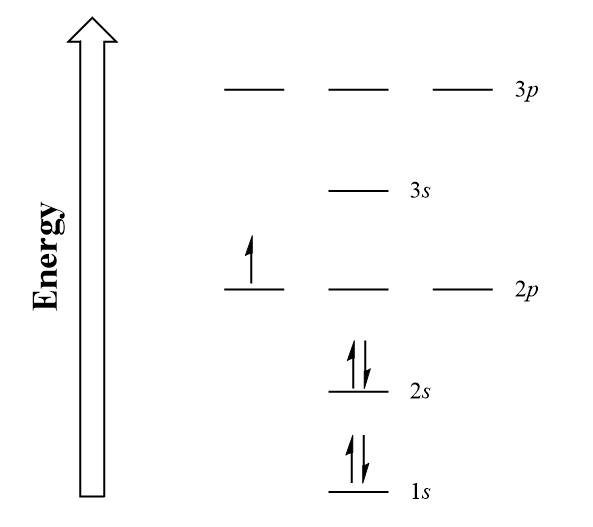
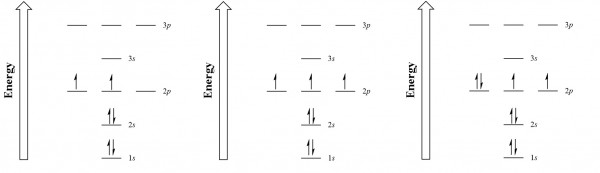
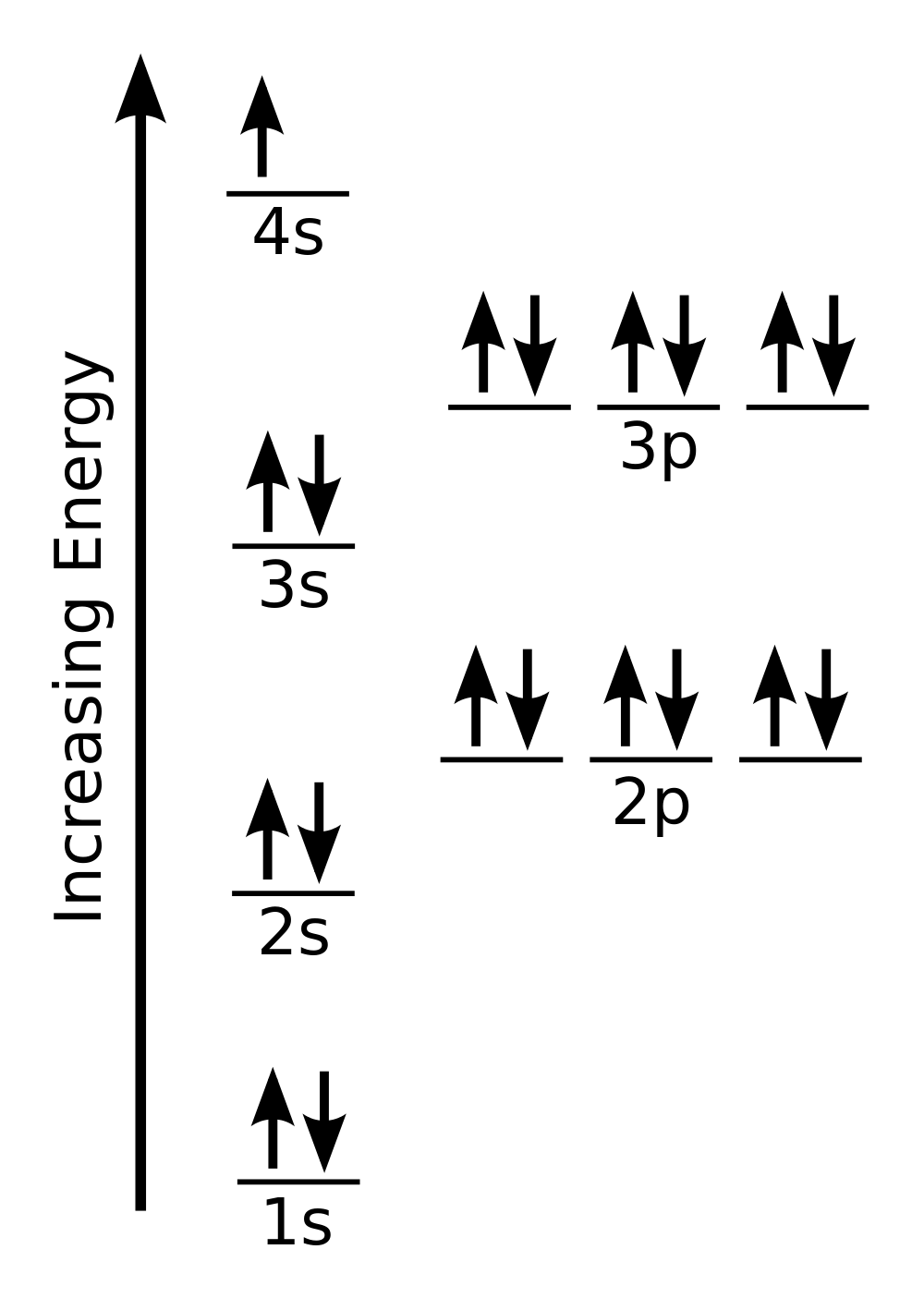
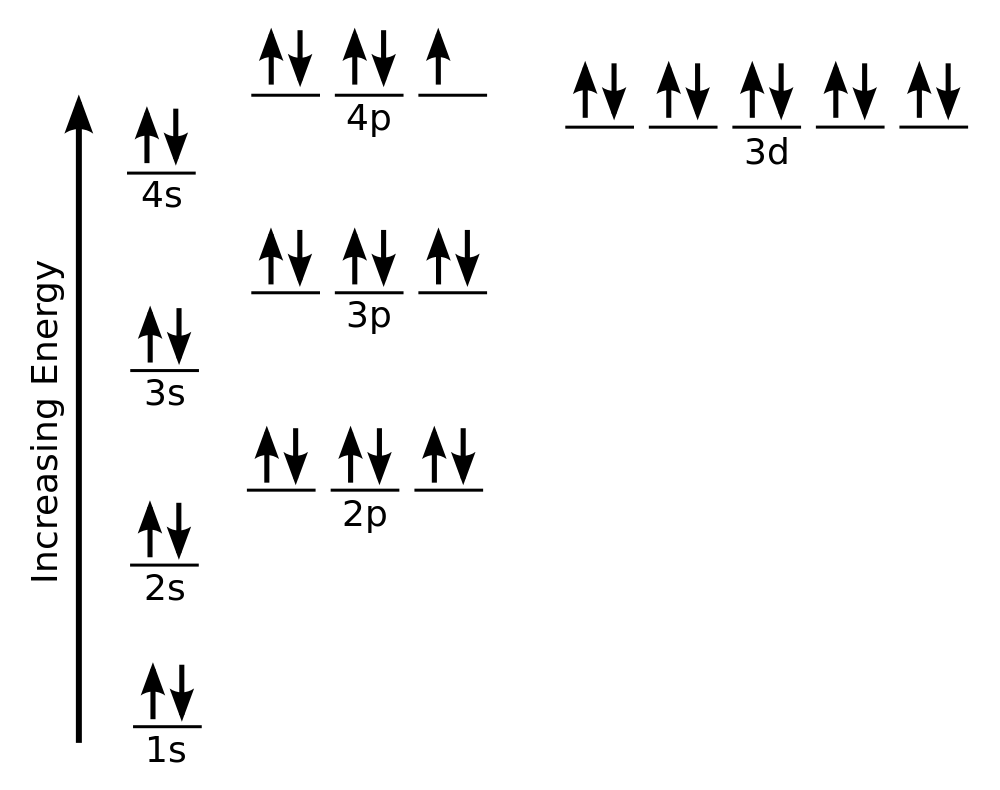
| aufbau principle | (German for “building up”): electrons fill the lowest energy orbitals first. | Organization of Electrons in Atoms |
| autoionization constant of water (Kw) | The product of the hydrogen ion and hydroxide ion concentrations | Autoionization of Water |
| autoionization of water | Water molecules act as acids (proton donors) and bases (proton acceptors) with each other to a tiny extent in all aqueous solutions | Autoionization of Water |
| Avogadro’s law | A gas law that relates number of particles to volume | Other Gas Laws |
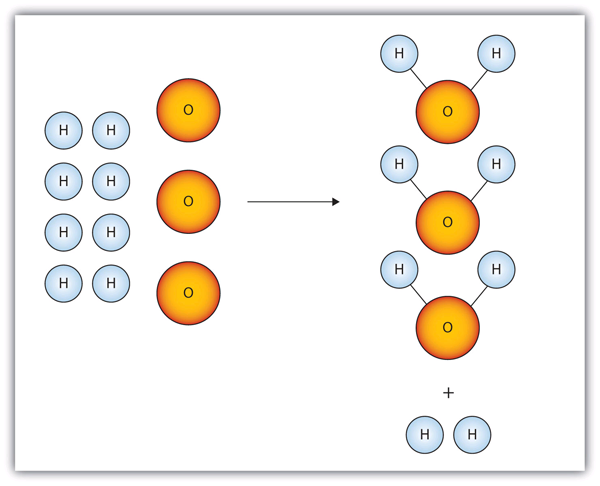
| balanced chemical equation | A condition when the reactants and products of a chemical equation have the same number of atoms of all elements present | The Chemical Equation |
| base | A compound that increases the amount of OH− ions in an aqueous solution | Neutralization Reactions |
| basic salt | An ionic compound whose aqueous solution is slightly basic | Strong and Weak Acids and Bases and Their Salts |
| becquerel (Bq) | A unit of radioactivity equal to 1 decay per second | Units of Radioactivity |
| beta particle | A type of radioactive emission equivalent to an electron | Radioactivity |
| boiling (or vaporization) | The process of a liquid becoming a gas | Phase Transitions: Melting, Boiling and Subliming |
| boiling point | The characteristic temperature at which a liquid becomes a gas | Phase Transitions: Melting, Boiling and Subliming |
| boiling point elevation | The increase of a solution’s boiling point because of the presence of solute | Colligative Properties of Solutions |
| boiling point elevation constant (Kb) | The constant that relates the molality concentration of a solution and its boiling point change | Colligative Properties of Solutions |
| bond energy | The approximate amount of energy needed to break a covalent bond | Other Aspects of Covalent Bonding |
| bond order | A method of evaluating bond strength | Molecular Orbitals |
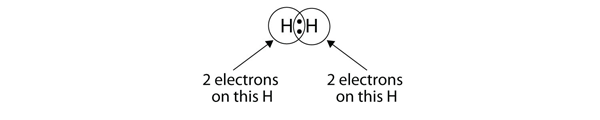
| bonding electron pair | A pair of electrons that makes a covalent bond | Covalent Bonds |
| bonding molecular orbital | The lower energy molecular orbital generated by constructive combination of atomic orbitals | Molecular Orbitals |
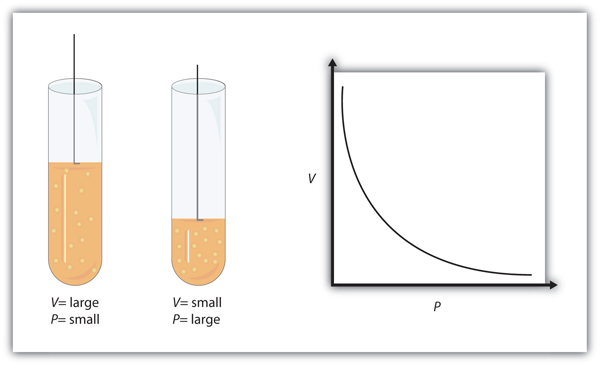
| Boyle’s law | A gas law that relates pressure and volume at constant temperature and amount | Gas Laws |
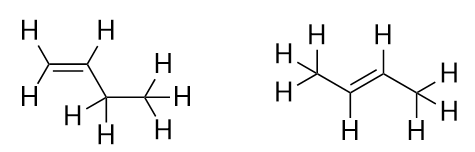
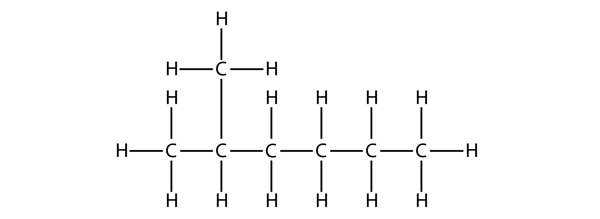
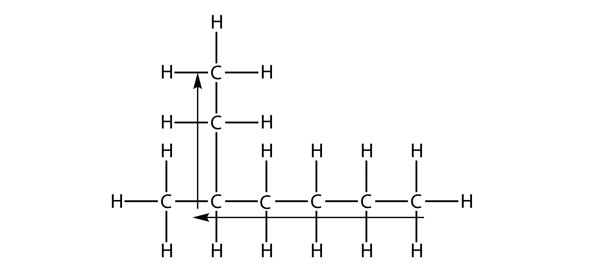
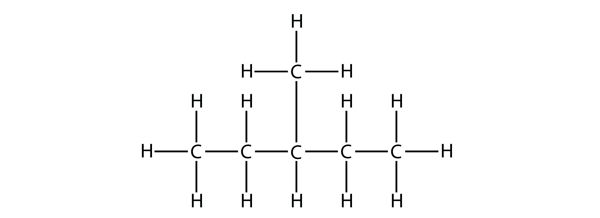
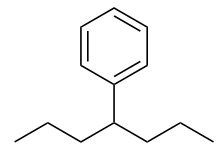
| branched hydrocarbons | A carbon compound that is not a straight chain, having substituents appended to the longest chain | Branched Hydrocarbons |
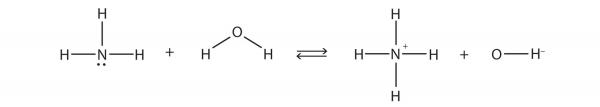
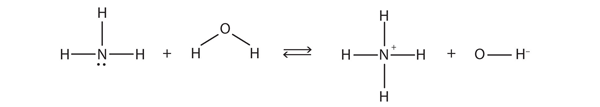
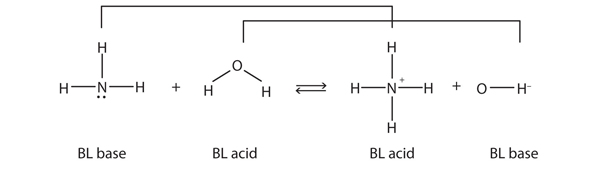
| Brønsted-Lowry acid | Any species that can donate a proton to another molecule | Brønsted-Lowry Acids and Bases |
| Brønsted-Lowry base | Any species that can accept a proton from another molecule | Brønsted-Lowry Acids and Bases |
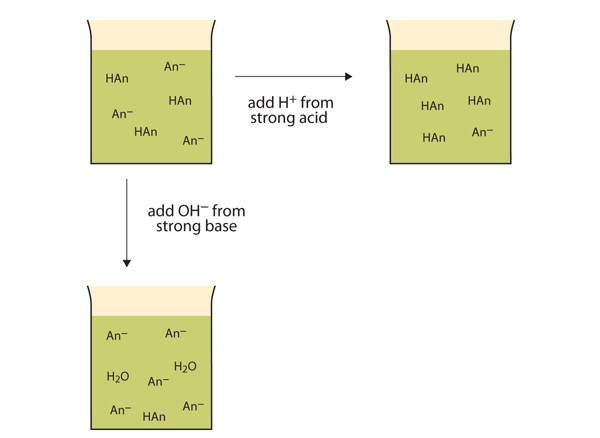
| buffer | A solution that resists dramatic changes in pH | Buffers |
| buffer capacity | The amount of strong acid or base a buffer can counteract | Buffers |
| burette or buret | A precisely calibrated volumetric delivery tube | Acid-Base Titrations |
| calorie | A unit of energy measurement originally defined in terms of warming up a given quantity of water. 1 cal = 4.184 J | Energy |
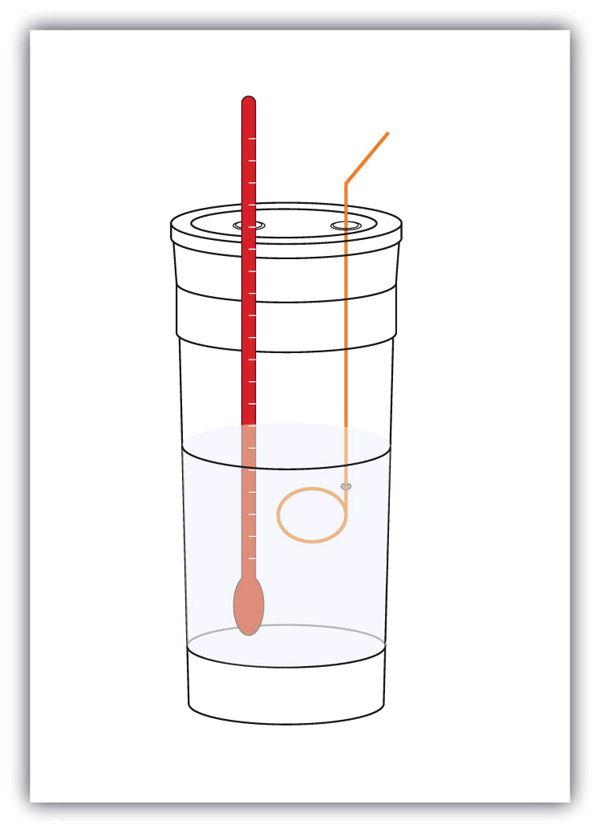
| calorimeter | A container used to measure the heat of a chemical reaction | Enthalpy and Chemical Reactions |
| calorimetry | The process of measuring enthalpy changes for chemical reactions | Enthalpy and Chemical Reactions |
| capillary action | The behavior of a liquid in narrow surfaces due to differences in adhesion and cohesion | Properties of Liquids |
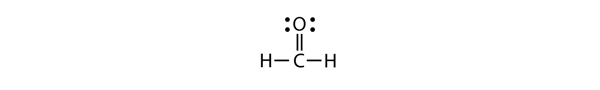
| carbonyl group | A functional group where an O atom and a C atom are joined with a double bond | Other Oxygen-Containing Functional Groups |
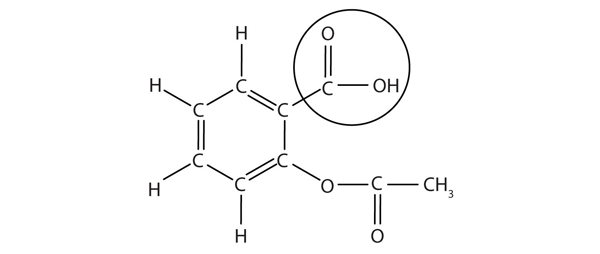
| carboxyl group | A functional group composed of a carbonyl group and an OH group | Other Oxygen-Containing Functional Groups |
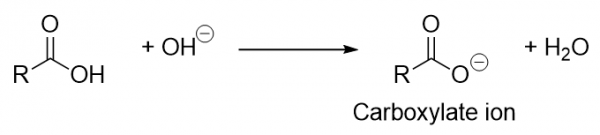
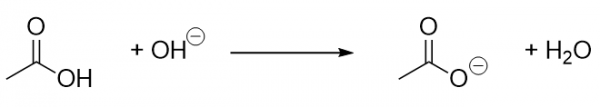
| carboxylate ion | A negatively charged ion derived from a carboxylic acid | Other Oxygen-Containing Functional Groups |
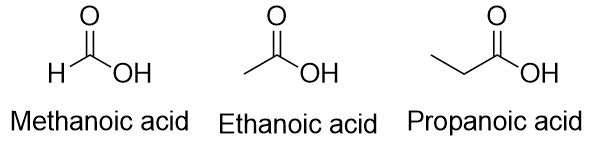
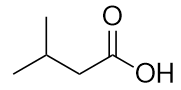
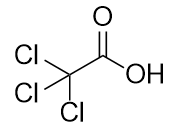
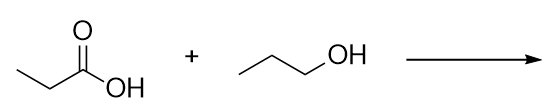
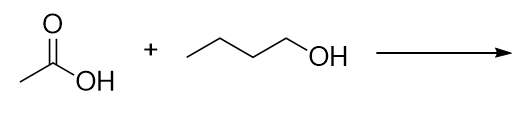
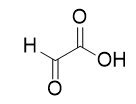
| carboxylic acid | A molecule with a carboxyl group | Other Oxygen-Containing Functional Groups |
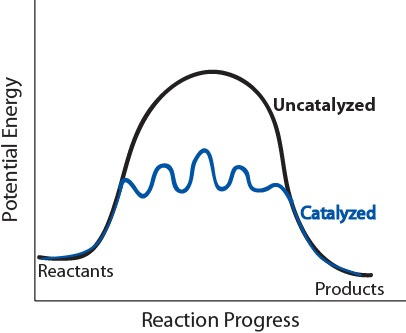
| catalyst | A substance that increases the speed of a reaction | Shifting Equilibria: Le Chatelier’s Principle |
| catalyst | A substance thaty accelerates a reaction by participating in it without being consumed | Factors that Affect the Rate of Reactions |
| catalyst | A substance that lowers the activation energy of a specific reaction by providing an alternate reaction pathway | Catalysis |
| cathode | The half cell that contains the reduction reaction | Applications of Redox Reactions: Voltaic Cells |
| cation | A species with an overall positive charge | Ions and Ionic Compounds |
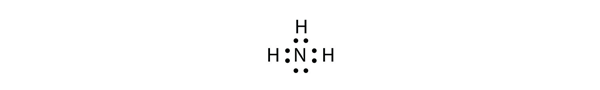
| central atom | The atom in the center of a molecule | Covalent Bonds |
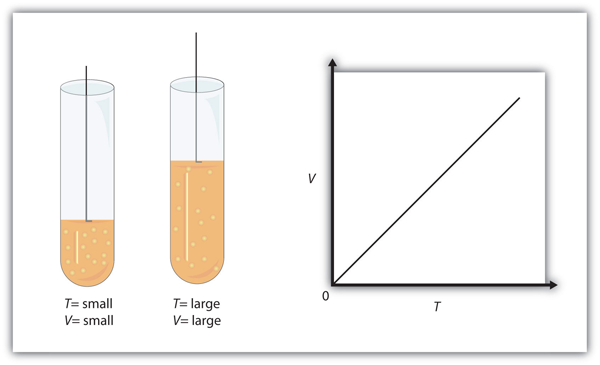
| Charles’s law | A gas law that relates volume and temperature at constant pressure and amount | Gas Laws |
| chemical bond | The connection between two atoms in a molecule | Molecules an Chemical Nomenclature |
| chemical change | The process of demonstrating a chemical property | Some Basic Definitions |
| chemical equation | A concise way of representing a chemical reaction | The Chemical Equation |
| chemical equilibrium | The point at which forward and reverse chemical reactions balance each other’s progress | Chemical Equilibrium |
| chemical nomenclature | A very specific system for naming compounds, in which unique substances get unique names | Molecules an Chemical Nomenclature |
| chemical property | A characteristic that describes how matter changes form in the presence of other matter | Some Basic Definitions |
| chemistry | The study of the interactions of matter with other matter and with energy | Introduction |
| coefficient | The part of a number in scientific notation that is multiplied by a power of 10 | Expressing Numbers |
| coefficient | A number in a chemical equation indicating more than one molecule of the substance | The Chemical Equation |
| cohesion | The tendency of a substance to interact with itself | Properties of Liquids |
| colligative property | A property of solutions related to the fraction that the solute particles occupy in the solution, not their identity | Colligative Properties of Solutions |
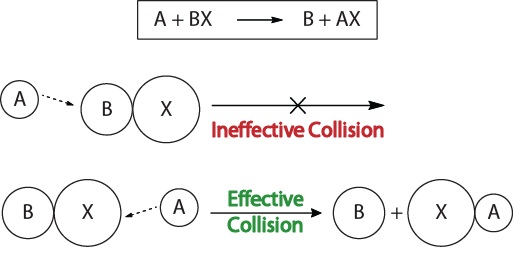
| collision theory | The theory that reactions occur when reactant molecules “effectively collide” | Factors that Affect the Rate of Reactions |
| combined gas law | A gas law that combines pressure, volume, and temperature | Other Gas Laws |
| combustion reaction | A chemical reaction in which a reactant combines with oxygen to produce oxides of all other elements as products | Composition, Decomposition, and Combustion Reactions |
| complete ionic equation | A chemical equation in which the dissolved ionic compounds are written as separated ions | Ionic Equations: A Closer Look |
| composition reaction | A chemical reaction in which a single substance is produced from multiple reactants | Composition, Decomposition, and Combustion Reactions |
| compound | A combination of more than one element | Some Basic Definitions |
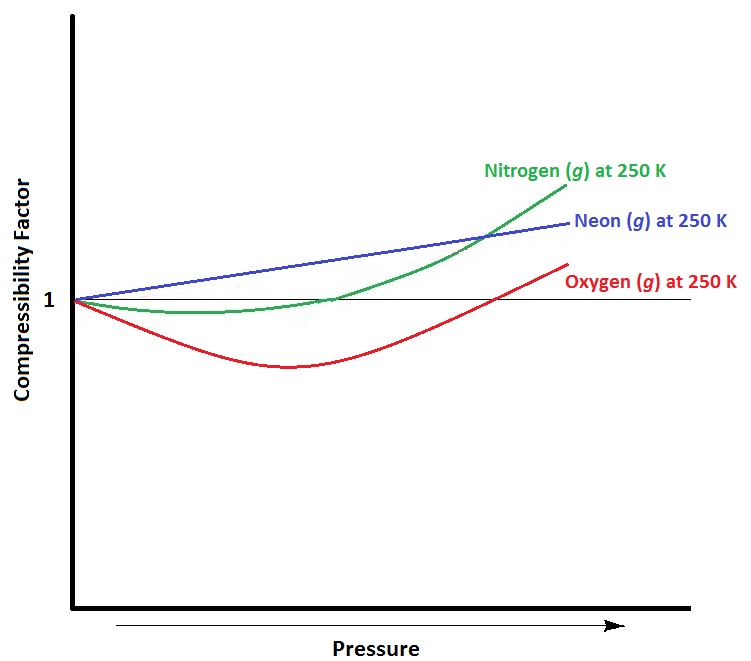
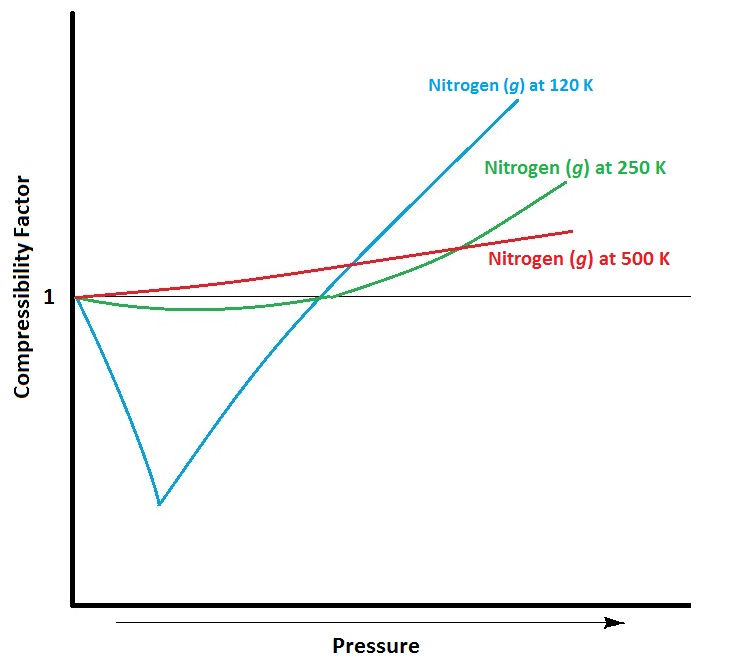
| compressibility factor | A measure of the extent of deviation from ideal gas behaviour | Real Gases |
| concentrated solution | A solution with a lot of solute | Some Definitions |
| concentration | How much solute is dissolved in a given amount of solvent | Some Definitions |
| concentration (verb) | The removal of solvent, which increases the concentration of the solute in the solution | Dilutions and Concentrations |
| condensation | The process of a gas becoming a liquid | Phase Transitions: Melting, Boiling and Subliming |
| condensed structure | A listing of the atoms bonded to each C atom in a chain | Hydrocarbons |
| conjugate acid-base pair | Two species whose formulas differ by only a hydrogen ion | Brønsted-Lowry Acids and Bases |
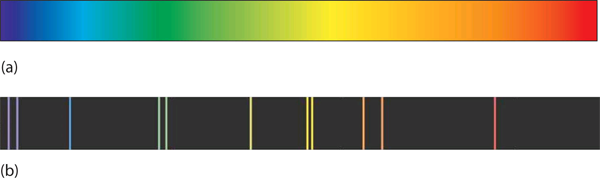
| continuous spectrum | An image that contains all colors of light | Quantum Numbers for Electrons |
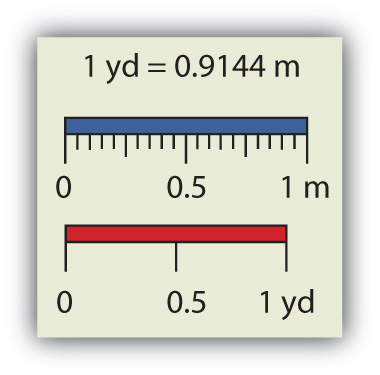
| conversion factor | A fraction that can be used to convert a quantity from one unit to another | Converting Units |
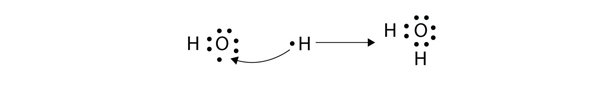
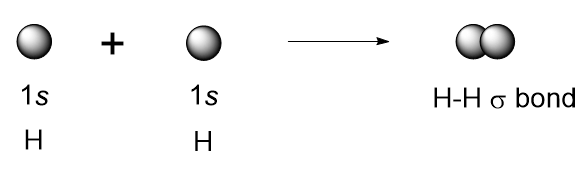
| covalent bond | A chemical bond formed by two atoms sharing electrons | Covalent Bonds |
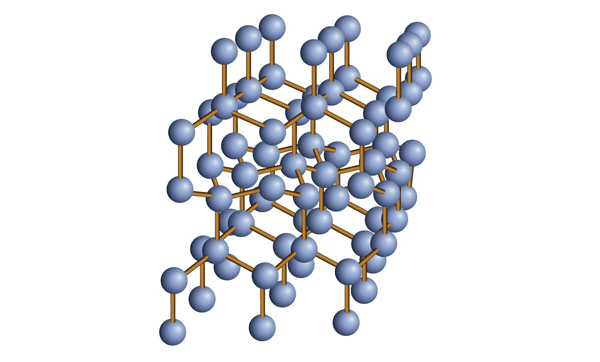
| covalent network solids | A crystalline solid composed of atoms of one or more elements that are covalently bonded together in a seemingly never-ending fashion | Solids |
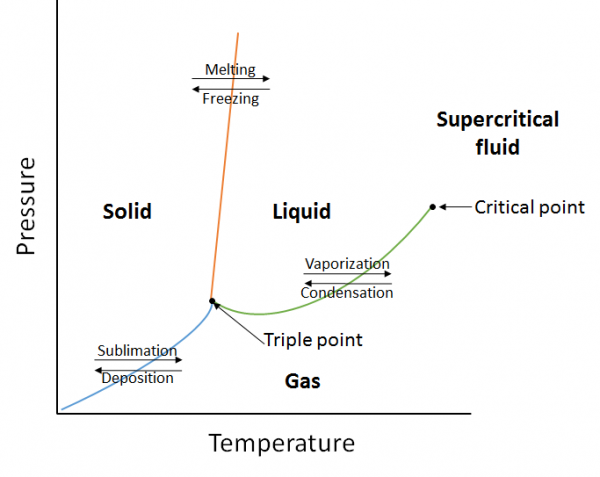
| critical point | The point at the highest temperature and pressure at which liquids and gases remain distinguishable | Properties of Liquids |
| crystalline solid | A solid with a regular, repeating three-dimensional structure | Solids |
| curie | A unit of radioactivity equal to 3.7×1010 decays/s | Units of Radioactivity |
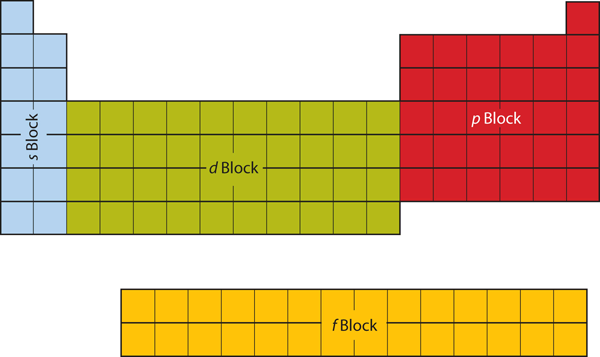
| d block | The columns of the periodic table in which d subshells are being occupied | Electronic Structure and the Periodic Table |
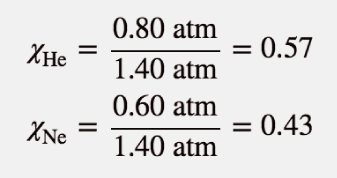
| Dalton’s law of partial pressures | The total pressure of a gas mixture, Ptot, is equal to the sum of the partial pressures of the components, Pi | Gas Mixtures |
| daughter isotope | The product left over from the parent isotope in a nuclear equation | Radioactivity |
| decomposition reaction | A chemical reaction in which a single substance becomes more than one substance | Composition, Decomposition, and Combustion Reactions |
| degrees | The unit of temperature scales | Other Units: Temperature and Density |
| density | A physical property defined as a substance’s mass divided by its volume | Other Units: Temperature and Density |
| deposition | The process of a gas becoming a solid | Phase Transitions: Melting, Boiling and Subliming |
| derived unit | A unit that is a product or a quotient of a fundamental unit | Expressing Units |
| diatomic molecule | A molecule with only two atoms | Molecules an Chemical Nomenclature |
| diffusion | The movement of gas molecules through one or more additional types of gas via random molecular motion | Molecular Effusion and Diffusion |
| dilute | A solution with very little solute | Some Definitions |
| dilution | The addition of solvent, which decreases the concentration of the solute in the solution | Dilutions and Concentrations |
| dilution equation | The mathematical formula for calculating new concentrations or volumes when a solution is diluted or concentrated | Dilutions and Concentrations |
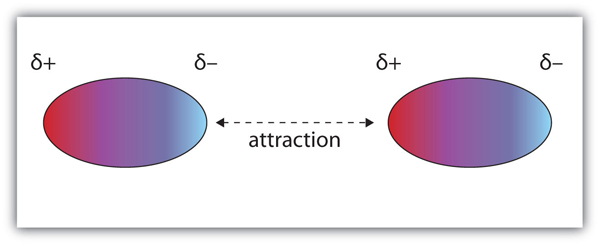
| dipole-dipole interactions | An intermolecular force caused by molecules with a permanent dipole | Intermolecular Forces |
| dispersion force (or London dispersion force) | An intermolecular force caused by the instantaneous position of an electron in a molecule | Intermolecular Forces |
| dissociation | The process of an ionic compound separating into ions when it dissolves | Ionic Equations: A Closer Look |
| double bond | A covalent bond composed of two pairs of bonding electrons | Covalent Bonds |
| double-replacement reaction | A chemical reaction in which parts of two ionic compounds are exchanged | Types of Chemical Reactions: Single- and Double-Displacement Reactions |
| dry cell | A modern battery that does not contain large amounts of aqueous solution | Applications of Redox Reactions: Voltaic Cells |
| dynamic equilibrium | When a process still occurs but the opposite process also occurs at the same rate so that there is no net change in the system. | Properties of Liquids |
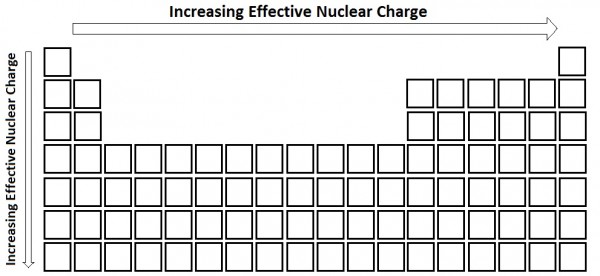
| effective nuclear charge (Zeff) | The net nuclear charge feld by valence electrons | Periodic Trends |
| effusion | The movement of gas molecules from one container to another via a tiny hole | Molecular Effusion and Diffusion |
| electrodes | The cathode or anode of a voltaic cell | Applications of Redox Reactions: Voltaic Cells |
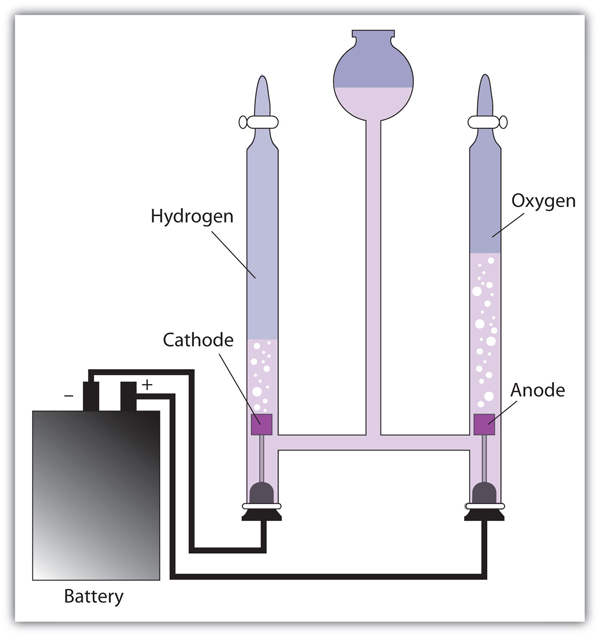
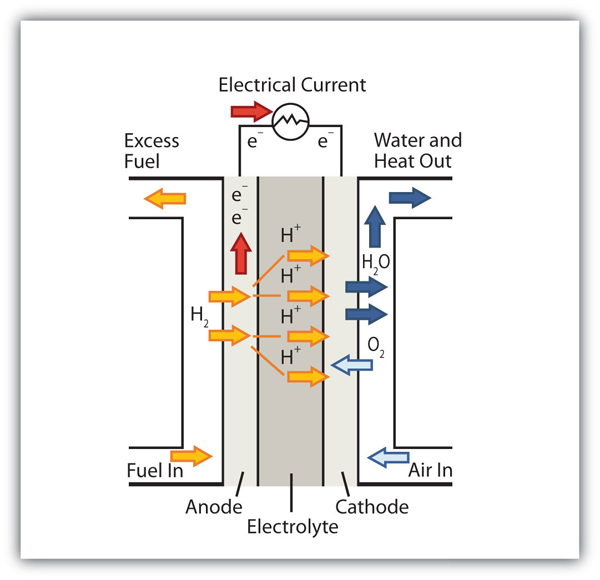
| electrolysis | The process of making a nonspontaneous redox reaction occur by forcing electricity into a cell | Electrolysis |
| electrolytic cell | A cell into which electricity is forced to make a nonspontaneous reaction occur | Electrolysis |
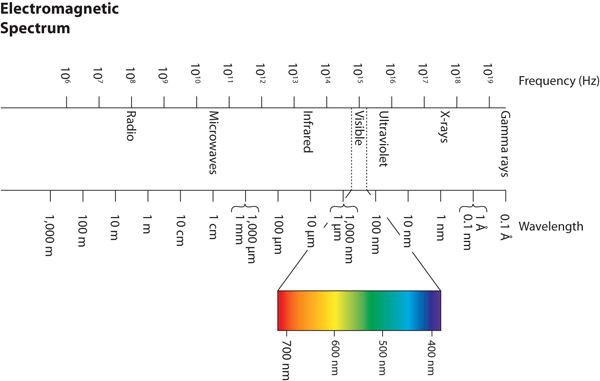
| electromagnetic spectrum | The full span of the possible wavelengths, frequencies, and energies of light | Light |
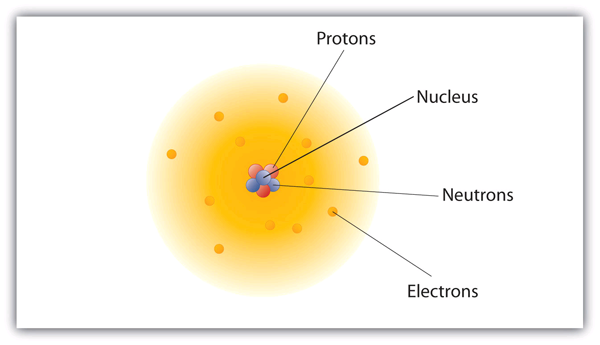
| electron | A tiny subatomic particle with a negative charge | Atomic Theory |
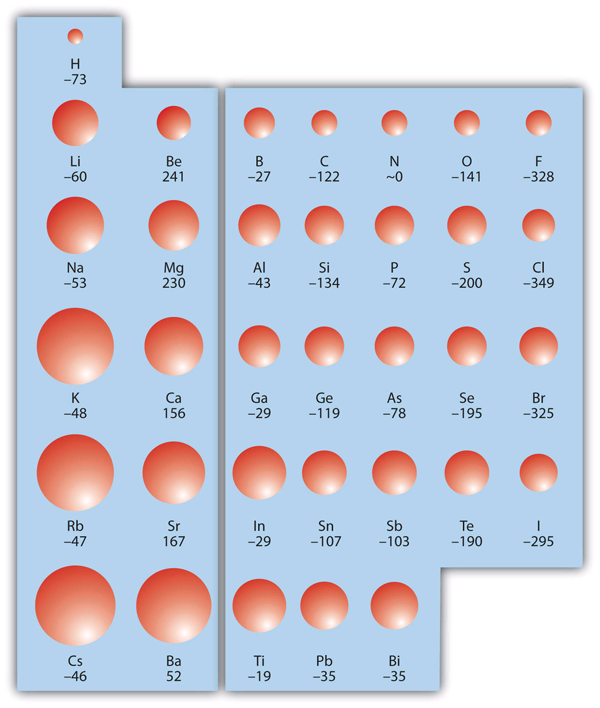
| electron affinity (EA) | The energy change when a gas-phase atom accepts an electron | Periodic Trends |
| electron configuration | A listing of the shell and subshells labels | Organization of Electrons in Atoms |
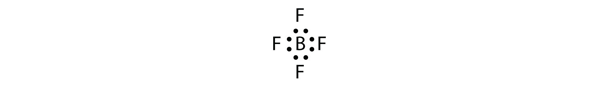
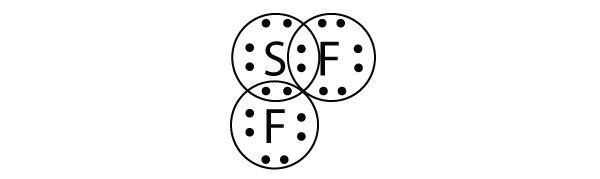
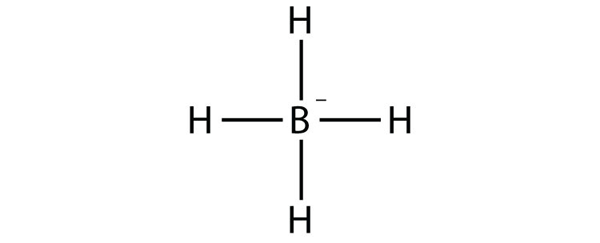
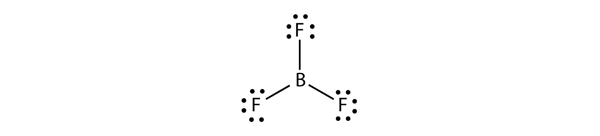
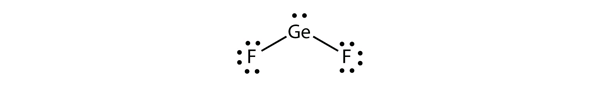
| electron deficient molecules | A molecule with less than eight electrons in the valence shell of an atom | Violations of the Octet Rule |
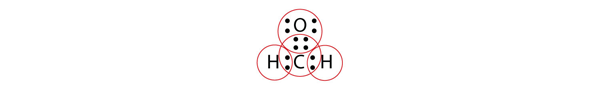
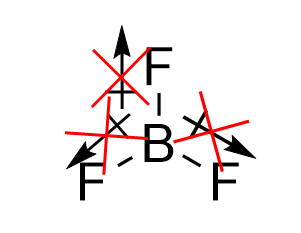
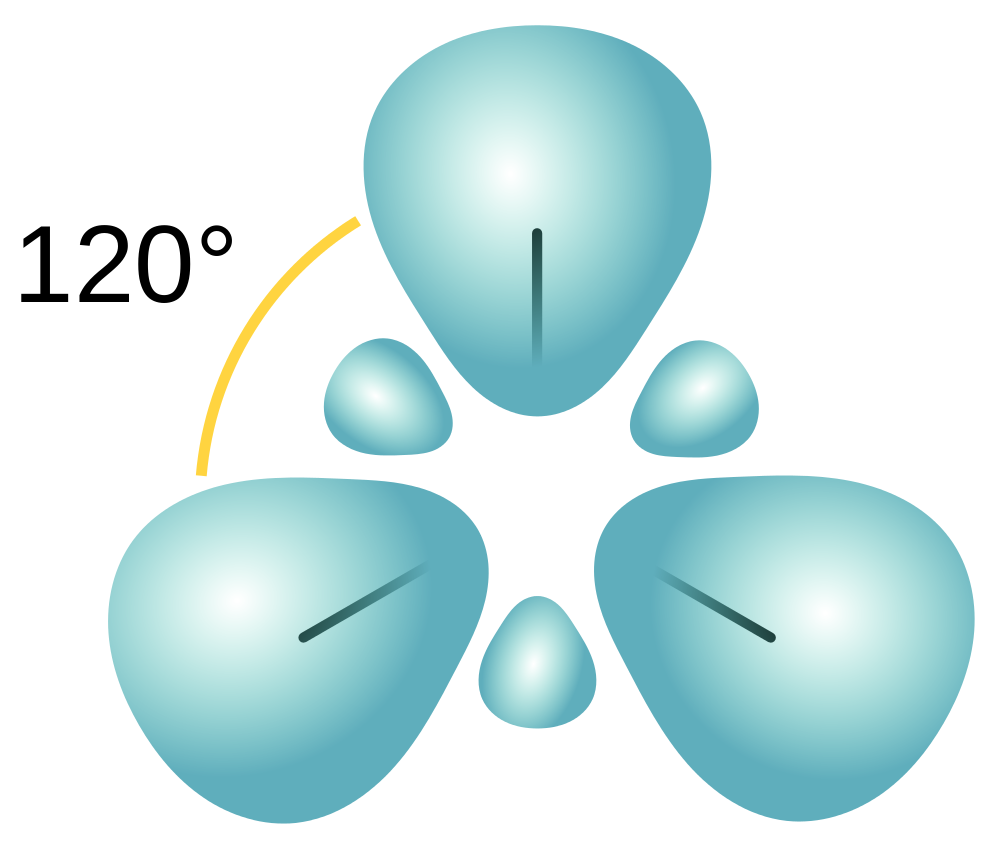
| electron group geometry | how electron groups (bonds and nonbonding electron pairs) are arranged | Molecular Shapes and Polarity |
| electron groups | A covalent bond of any type or a lone electron pair | Molecular Shapes and Polarity |
| electron shell | A term used to describe electrons with the same principal quantum number | Quantum Numbers for Electrons |
| electronegativity | A scale for judging how much atoms of any element attract electrons | Other Aspects of Covalent Bonding |
| electroplating | The deposition of a thin layer of metal on an object for protective or decorative purposes | Electrolysis |
| element | A substance that cannot be broken down into simpler chemical substances by ordinary chemical means | Some Basic Definitions |
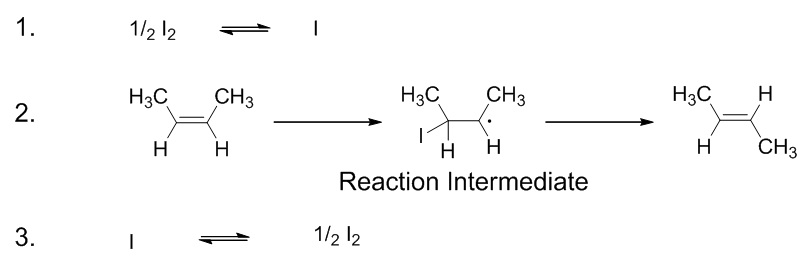
| elementary step | Each event that occurs in a chemical reaction as a result of an effective collision | Reaction Mechanisms |
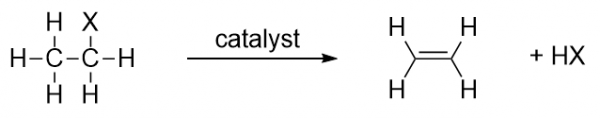
| elimination reaction | The removal of a functional group (either X or OH) and a H atom from an adjacent carbon | Alkyl halides and alcohols |
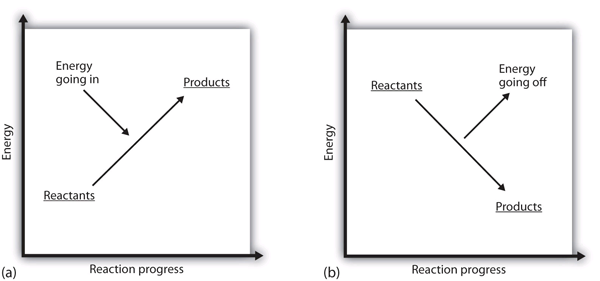
| endothermic | A chemical reaction that has a positive change in enthalpy | Enthalpy and Chemical Reactions |
| energy | The ability to do work. is the ability to do work | Energy |
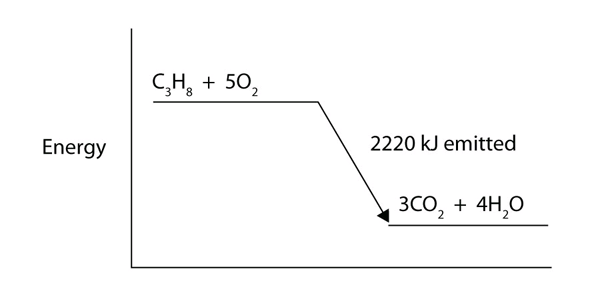
| enthalpy change | The heat of a process at constant pressure; denoted ΔH | Enthalpy and Chemical Reactions |
| enthalpy of formation | The enthalpy change for a formation reaction; denoted ΔHf. and is given the symbol ΔHf | Formation Reactions |
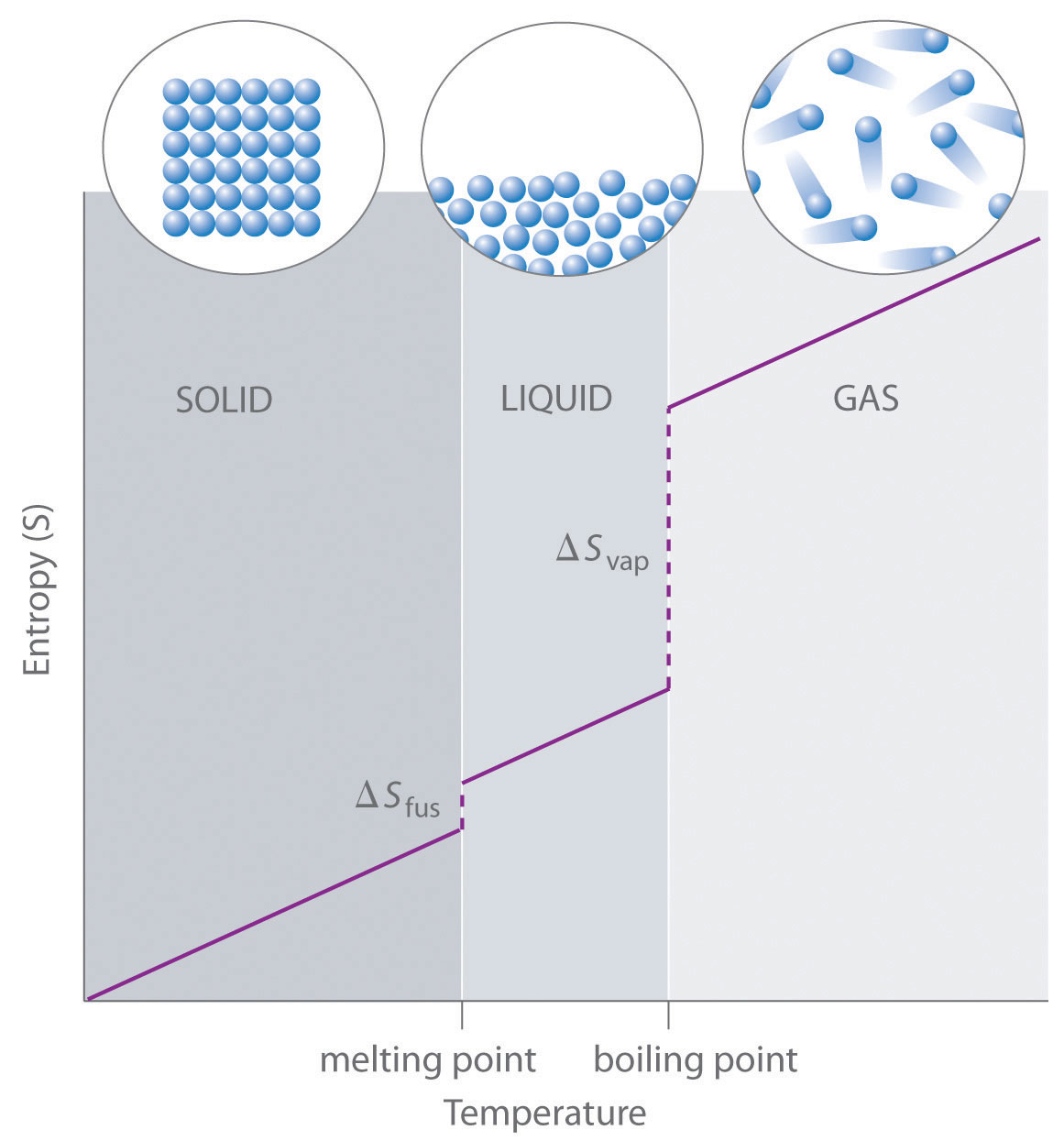
| enthalpy of fusion | The amount of energy needed to change from a solid to a liquid or from a liquid to a solid | Phase Transitions: Melting, Boiling and Subliming |
| enthalpy of sublimation | The amount of energy needed to change from a solid to a gas or from a gas to a solid | Phase Transitions: Melting, Boiling and Subliming |
| enthalpy of vaporization | The amount of energy needed to change from a liquid to a gas or from a gas to a liquid | Phase Transitions: Melting, Boiling and Subliming |
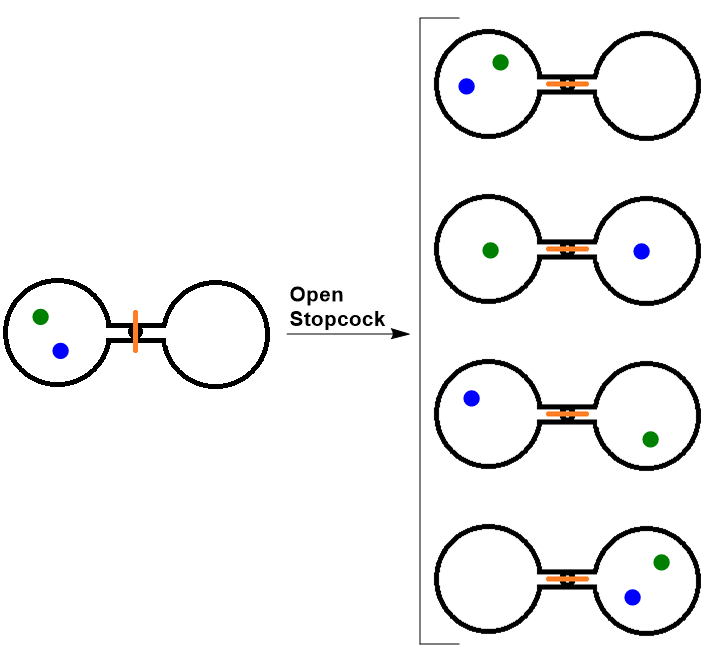
| entropy | The level of randomness (or disorder) of a system, or a measure of the energy dispersal of the molecules in the system | Entropy and the Second Law of Thermodynamics |
| enzyme | Protein molecules which serve to catalyze biochemical reactions | Catalysis |
| enzyme-substrate complex | The binding of substrate to the enzymatic active site | Catalysis |
| equilibrium constant (Keq) | A numerical value that relates to the ratio of products and reactants at equilibrium | The Equilibrium Constant |
| equivalence point | The point of the reaction when all the analyte has been reacted with the titrant | Acid-Base Titrations |
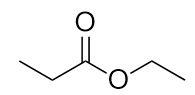
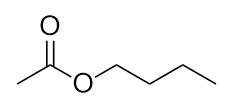
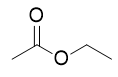
| ester group | A functional group made by combining a carboxylic acid with an alcohol | Other Oxygen-Containing Functional Groups |
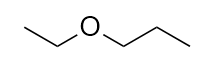
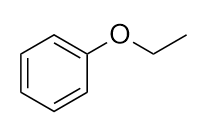
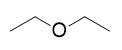
| ether group | A functional group that has an O atom attached to two organic groups | Other Oxygen-Containing Functional Groups |
| evaporation | The formation of a gas phase from a liquid at temperatures below the boiling point | Properties of Liquids |
| exact number | A number from a defined relationship that technically has an infinite number of significant figures | Converting Units |
| exothermic | A chemical reaction that has a negative change in enthalpy | Enthalpy and Chemical Reactions |
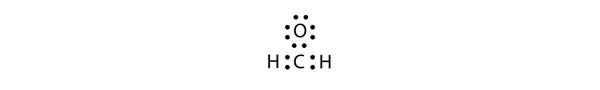
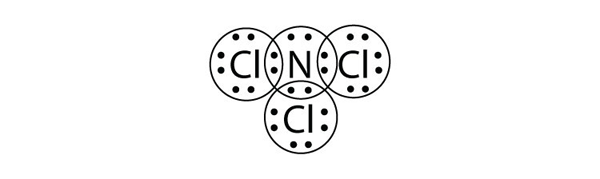
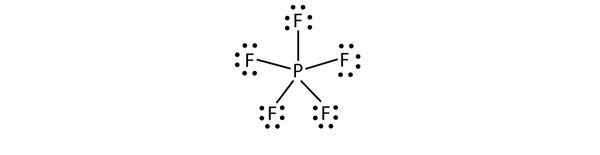
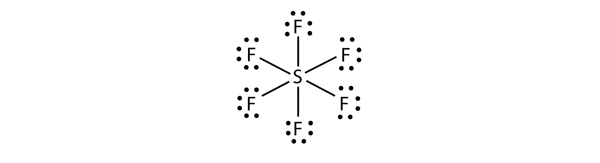
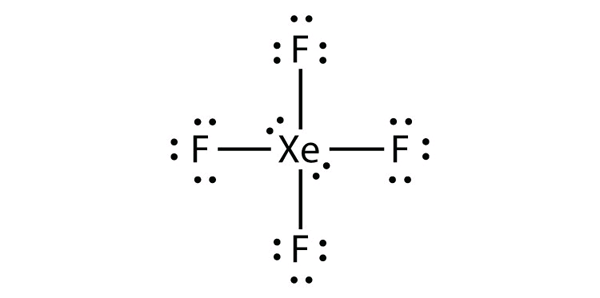
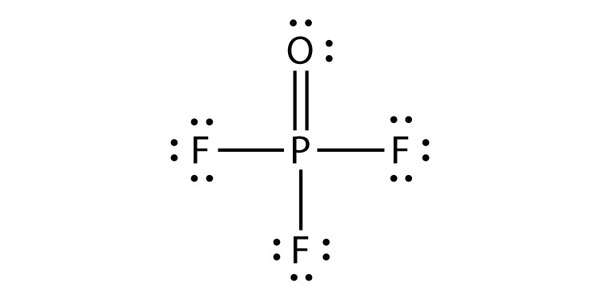
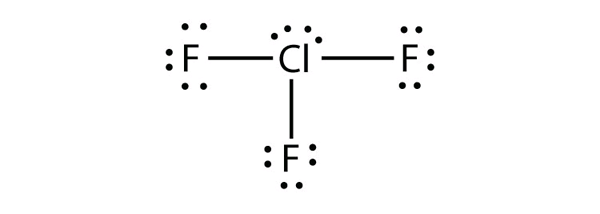
| expanded valence shell molecules | A molecule with more than eight electrons in the valence shell of an atom | Violations of the Octet Rule |
| experiment | A test of the natural universe to see if a guess (hypothesis) is correct | Chemistry as a Science |
| exponent | The raised number to the right of a 10 indicating the number of factors of 10 in the original number | Expressing Numbers |
| f block | The columns of the periodic table in which f subshells are being occupied | Electronic Structure and the Periodic Table |
| fission | The breaking apart of an atomic nucleus into smaller nuclei | Radioactivity |
| formation reaction | A chemical reaction that forms one mole of a substance from its constituent elements in their standard states | Formation Reactions |
| freezing point depression | The decrease of a solution’s freezing point because of the presence of solute | Colligative Properties of Solutions |
| freezing point depression constant (Kf) | The constant that relates the molality concentration of a solution and its freezing point change | Colligative Properties of Solutions |
| frequency | The number of cycles of light that pass a given point in one second | Light |
| frequency factor (A) | A factor that takes into account the frequency of reactions and the likelihood of correct molecular orientation | Activation Energy and the Arrhenius Equation |
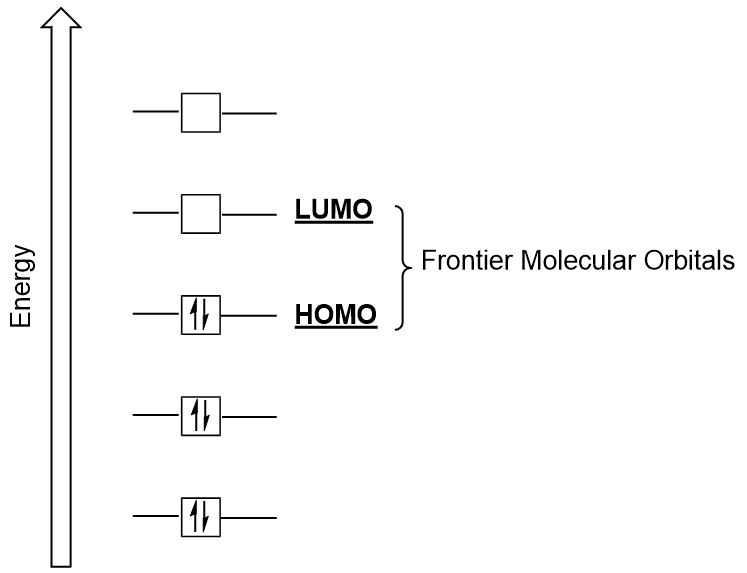
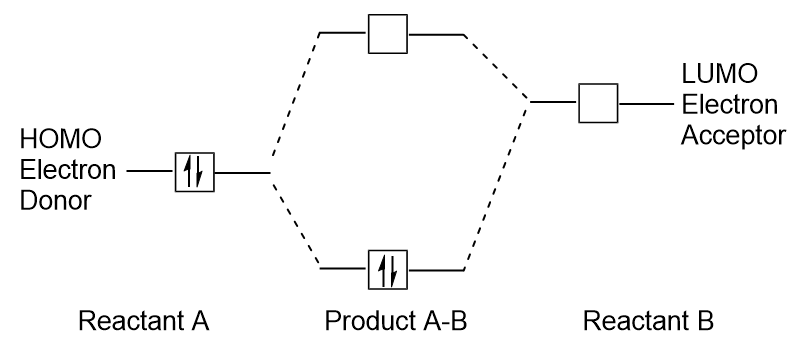
| frontier molecular orbitals | A term which refers to the HOMO and LUMO, the most likely orbitals to be involved in chemical reactions or processes | Molecular Orbitals |
| functional group | A collection of atoms or bonds with certain characteristic reactions | Alkyl halides and alcohols |
| fundamental units | One of the seven basic units of SI used in science | Expressing Units |
| gamma ray | A type of radioactive emission that is a very energetic form of electromagnetic radiation | Radioactivity |
| gas law | A simple mathematical formula that allows one to model, or predict, the behavior of a gas | Gas Laws |
| Gay-Lussac’s law | A gas law that relates pressure with absolute temperature | Other Gas Laws |
| Geiger counter | An electrical device that detects radioactivity | Units of Radioactivity |
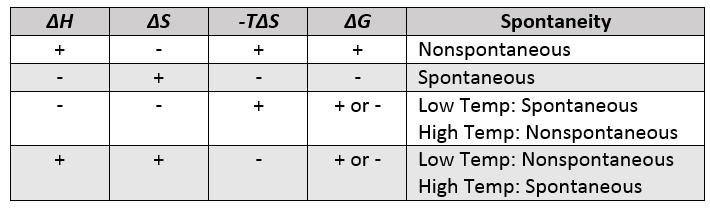
| Gibbs free energy (G) | A measure of spontaneity which incorporates both enthalpy and entropy | Gibbs Free Energy |
| Graham’s law of effusion | A law which relates the rate of effusion of a gas to the inverse of the square root of its molar mass | Molecular Effusion and Diffusion |
| gray (Gy) | A unit of radioactive exposure qual to 100 rad | Units of Radioactivity |
| half cell | A part of a voltaic cell that contains one half reaction | Applications of Redox Reactions: Voltaic Cells |
| half reaction | The individual oxidation or reduction reaction of a redox reaction | Balancing Redox Reactions |
| half reaction method | The method of balancing redox reactions by writing and balancing the individual half reactions | Balancing Redox Reactions |
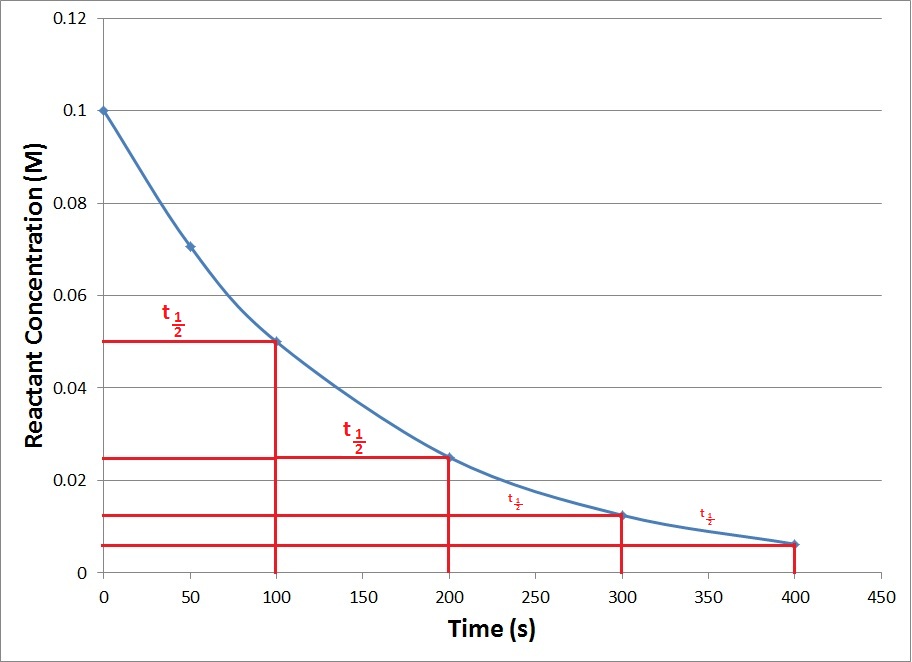
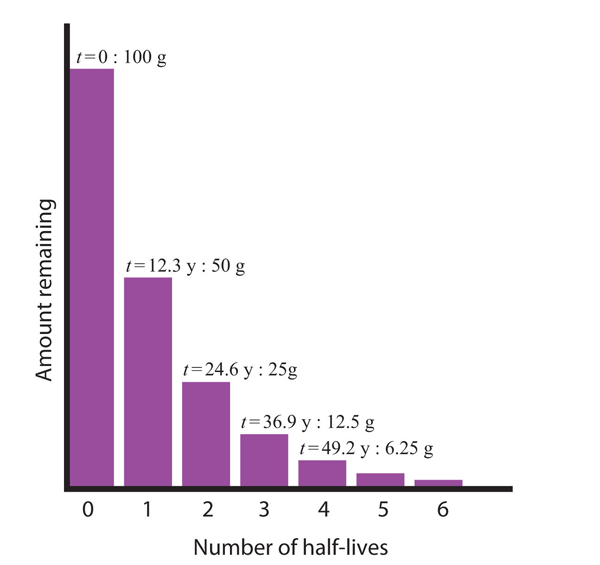
| half-life | The amount of time it takes for one-half of a radioactive isotope to decay | Half-Life |
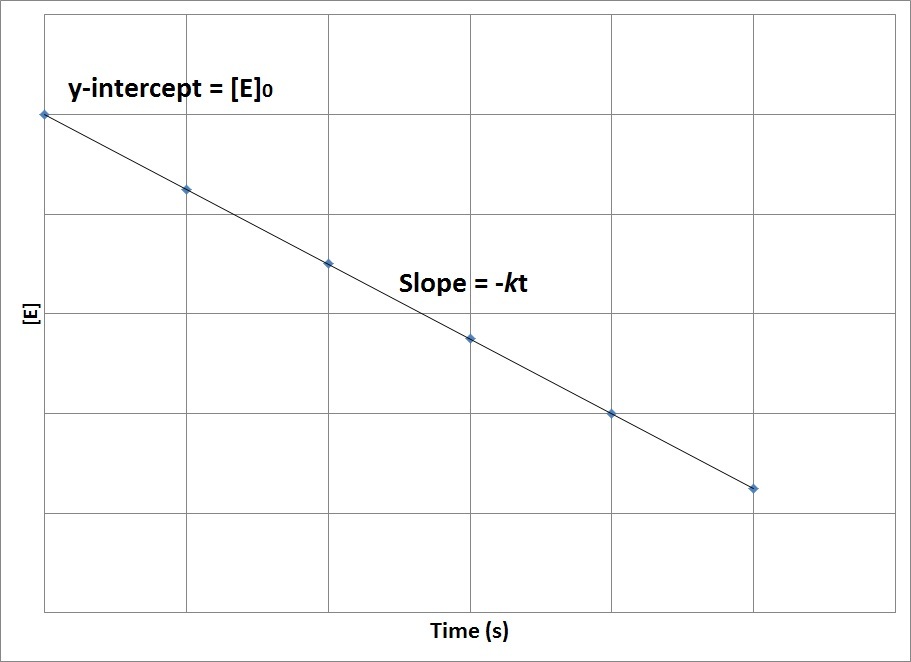
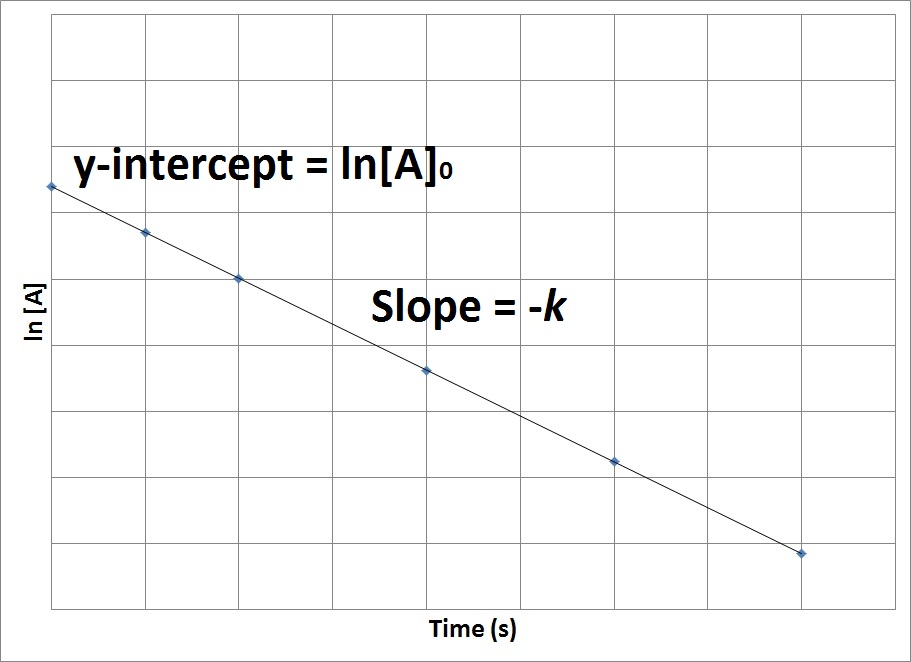
| half-life | The amount of time required for the concentration of a reactant to drop to one half of its initial concentration | Concentration-Time Relationships: Integrated Rate Laws |
| heat | The transfer of energy from one body to another due to a difference in temperature | Work and Heat |
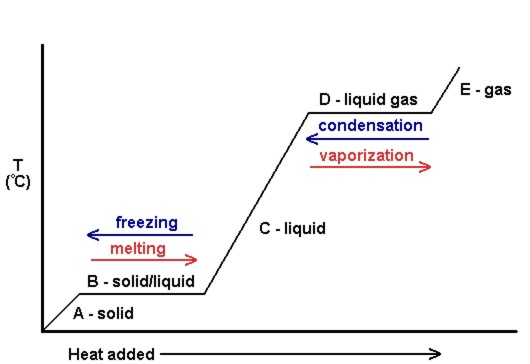
| heating curve | A plot of the temperature versus the amount of heat added | Phase Transitions: Melting, Boiling and Subliming |
| Hess’s law | When chemical equations are combined algebraically, their enthalpies can be combined in exactly the same way | Hess’s Law |
| heterogeneous catalyst | A catalyst that is in a different phase from one or more of the reactants | Catalysis |
| heterogeneous equilibrium | An equilibrium in which more than one phase of reactants or products is present | The Equilibrium Constant |
| heterogeneous mixture | A non-uniform combination of more than one substance | Some Basic Definitions |
| HOMO | The highest occupied molecular orbital | Molecular Orbitals |
| homogeneous catalyst | A catalyst that is present in the same phase as the reactant molecules | Catalysis |
| homogeneous mixture | A uniform mixture of more than one substance that behaves as a single substance | Some Basic Definitions |
| Hund’s rule | One electron is placed in each degenerate orbital before pairing electrons in the same orbital | Organization of Electrons in Atoms |
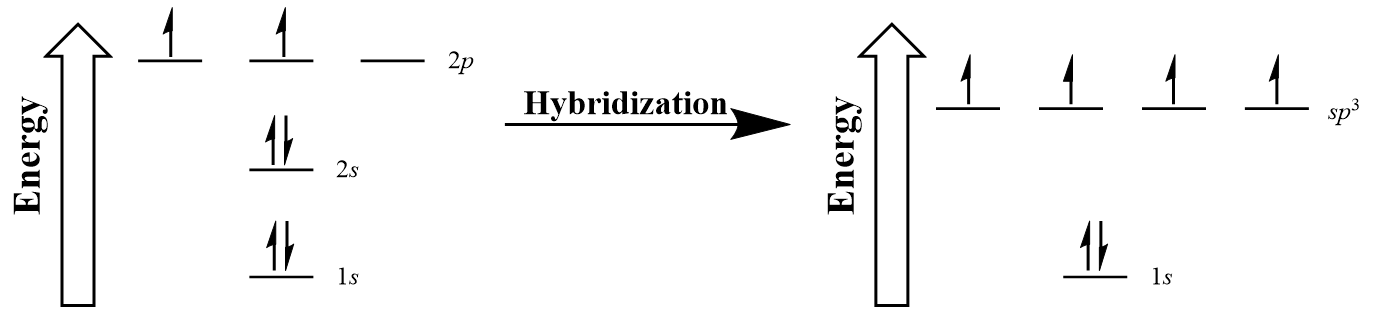
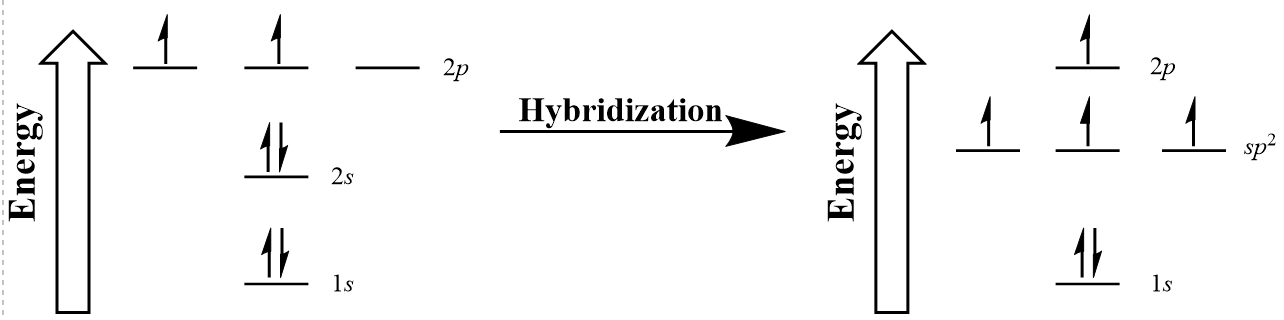
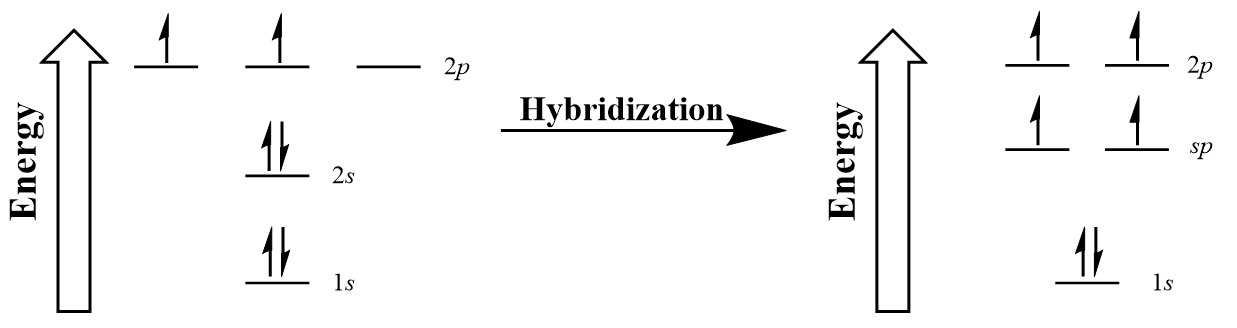
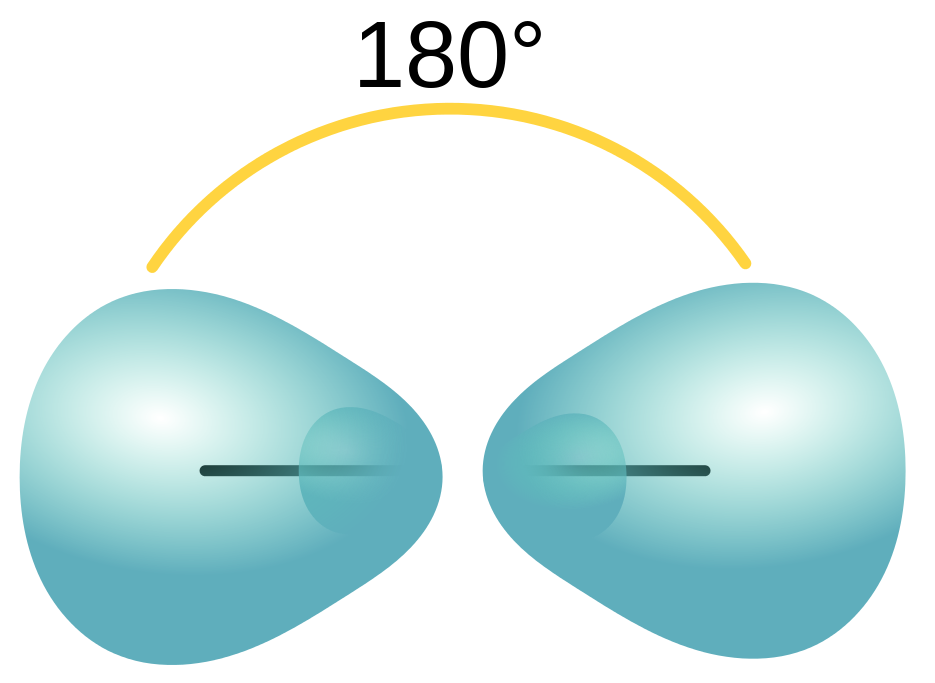
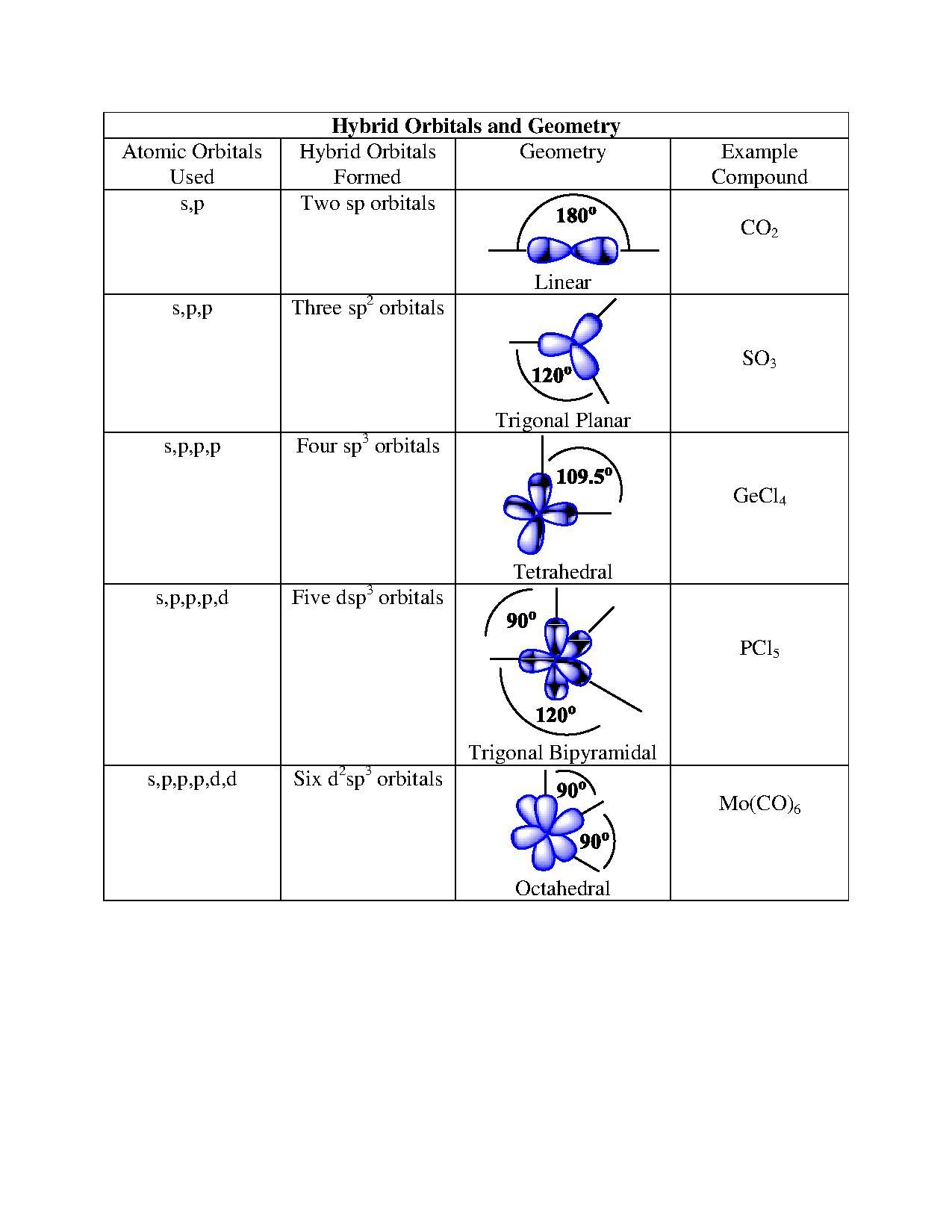
| hybridization | A mathematical mixing of atomic orbitals | Valence Bond Theory and Hybrid Orbitals |
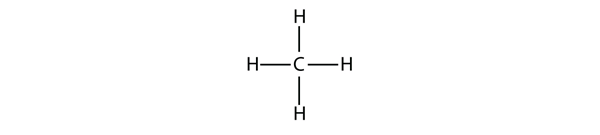
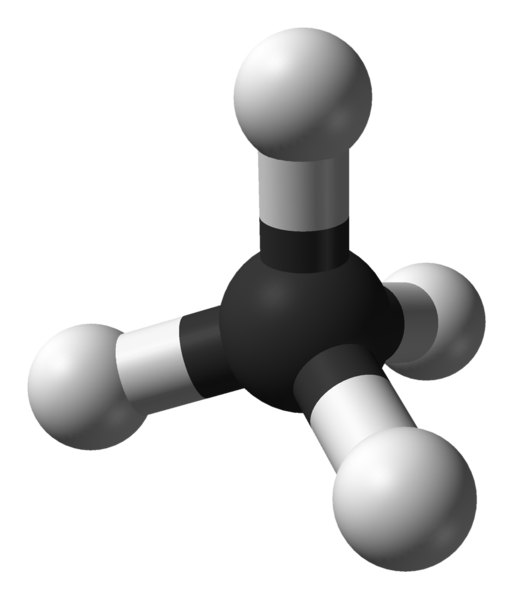
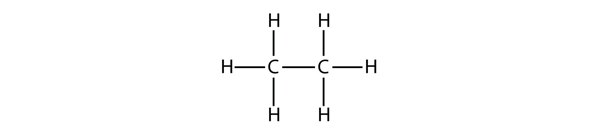
| hydrocarbons | An organic compound composed of carbon and hydrogen | Hydrocarbons |
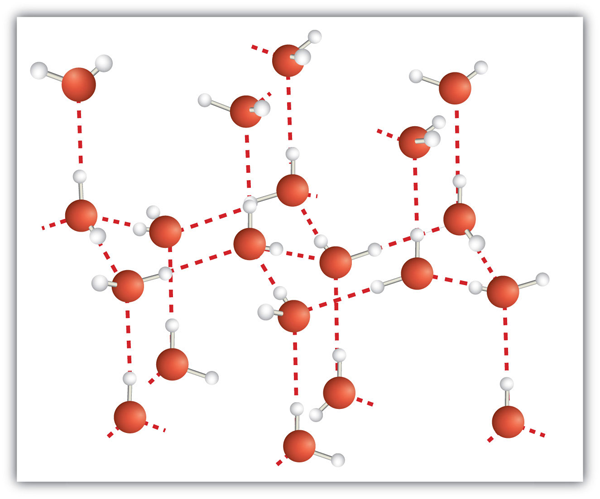
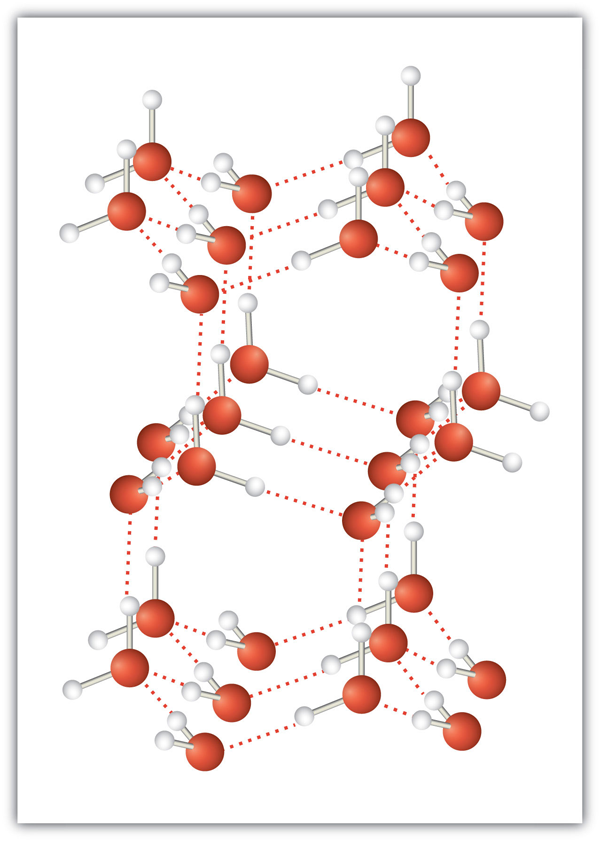
| hydrogen bonding | The very strong interaction between molecules due to H atoms being bonded to N, O, or F atoms | Intermolecular Forces |
| hydrogenation reaction | The reaction of hydrogen across a C–C double or triple bond, usually in the presence of a catalyst | Hydrocarbons |
| hydronium ion | The actual chemical species that represents a hydrogen ion in aqueous solution | Arrhenius Acids and Bases |
| hypothesis | An educated guess about how the natural universe works | Chemistry as a Science |
| hyrolysis | A reaction with water | Brønsted-Lowry Acids and Bases |
| ICE chart | A table used to calculate equilibria values featuring rows of initial, change and equlibria concentration | Calculating Equilibrium Constant Values |
| ideal gas | A gas that conforms exactly to the tenets of the kinetic molecular theory | Real Gases |
| ideal gas law | A gas law that relates all four independent physical properties of a gas under any conditions | The Ideal Gas Law and Some Applications |
| indicator | A substance whose color change indicates the equivalence point of a titration | Acid-Base Titrations |
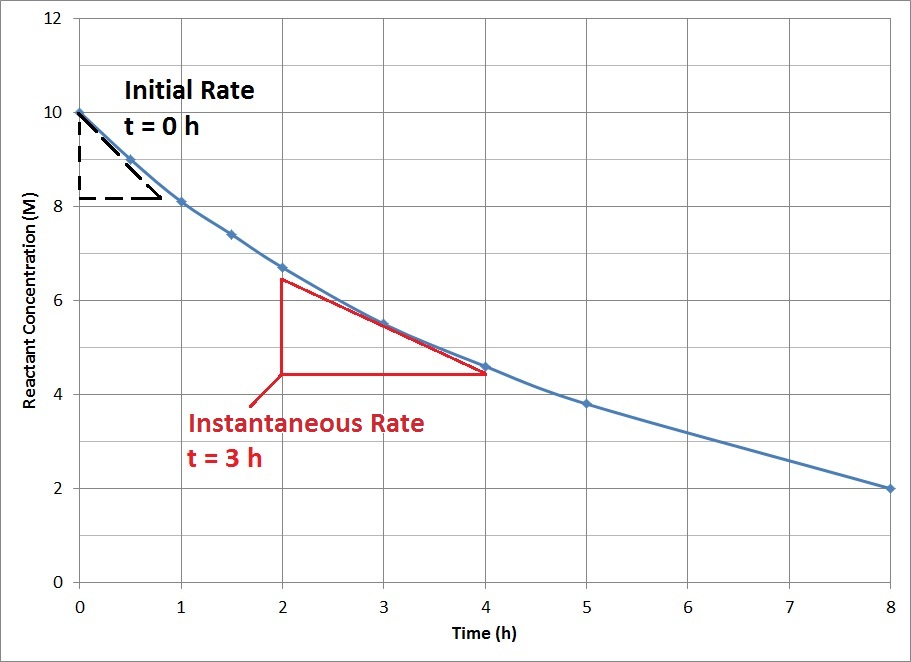
| initial rate | The instantaneous rate at the start of a reaction | Reaction Rates |
| initial rates method | A method to determine the rate law from the instantaneous reaction rate upon mixing the reactants | Rate Laws |
| instantaneous reaction rate | The rate of reaction at one instant in time | Reaction Rates |
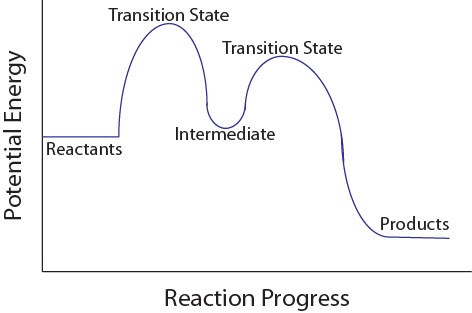
| intermediate | A chemical species does not appear in the overall balanced equation and is generated in one elementary step but used up in a subsequent step | Reaction Mechanisms |
| ion | A species with an overall electric charge | Ions and Ionic Compounds |
| ionic compound | A compound formed from positive and negative ions | Ions and Ionic Compounds |
| ionic formula | The chemical formula for an ionic compound | Ions and Ionic Compounds |
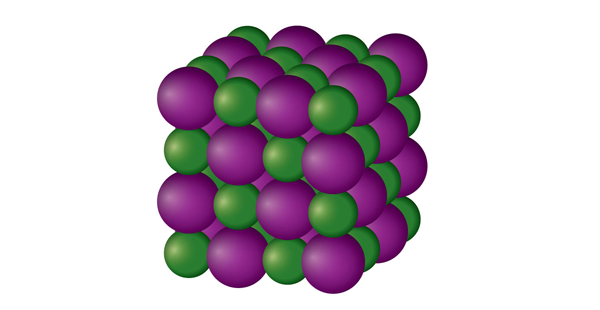
| ionic solid | A crystalline solid composed of ions | Solids |
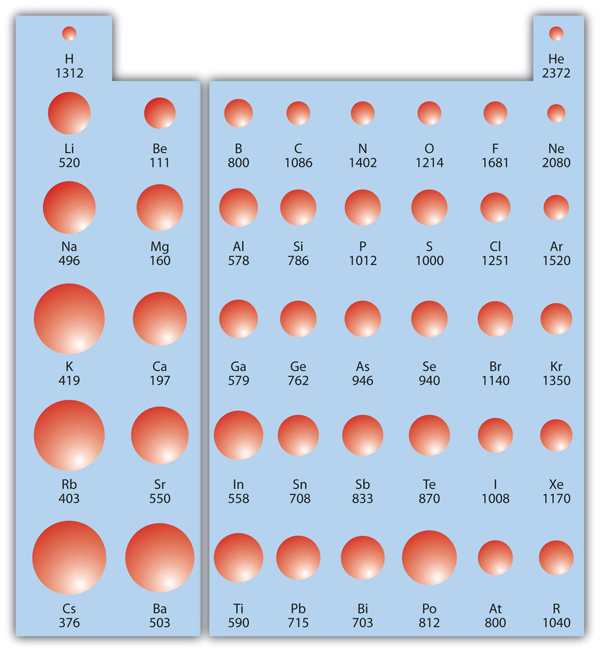
| ionization energy (IE) | The amount of energy required to remove an elec tron from an atom in the gas phase | Periodic Trends |
| isolated system | A system that does not allow a transfer of energy or matter into or out of the system | Energy |
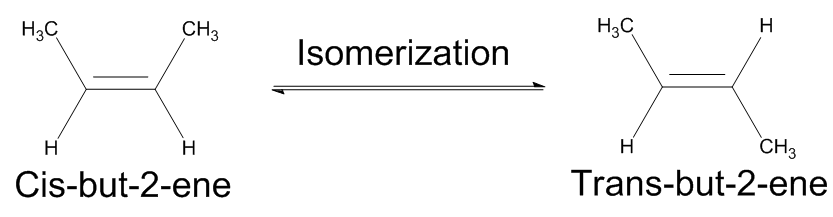
| isomer | A molecule with the same molecular formula as another molecule but a different structure | Hydrocarbons |
| isothermal | A process that does not change the temperature | Phase Transitions: Melting, Boiling and Subliming |
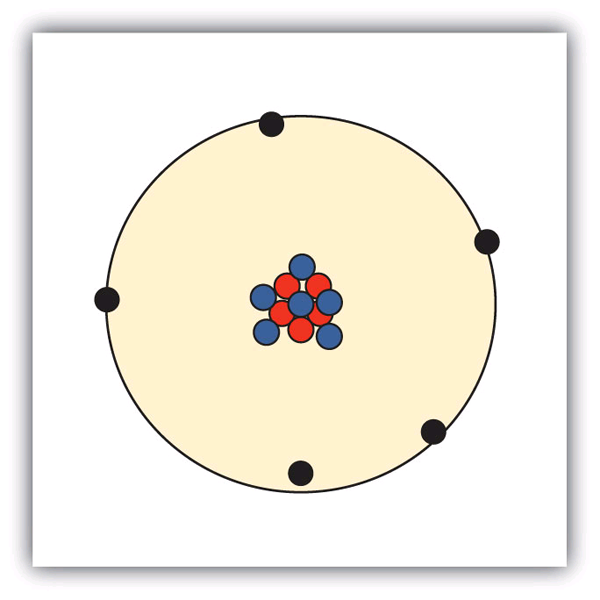
| isotopes | Atoms of the same element that have different numbers of neutrons | Atomic Theory |
| joule | The SI unit of energy | Energy |
| Kelvin scale | The fundamental unit of temperature in SI | Other Units: Temperature and Density |
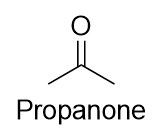
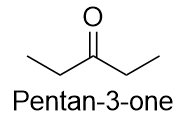
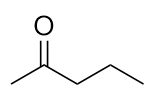
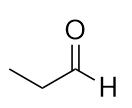
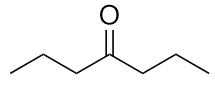
| ketone | A compound where the carbonyl carbon is attached to two carbon chains | Other Oxygen-Containing Functional Groups |
| kinetic energy | The energy due to motion | Kinetic-Molecular Theory of Gases |
| kinetic-molecular theory of gases | A model which helps us understand gases at the molecular level and their physical properties | Kinetic-Molecular Theory of Gases |
| kinetics | The study of reaction rate and the factors that can influence reaction rate | Introduction to Kinetics |
| law of conservation of energy | The total energy of an isolated system does not increase or decrease | Energy |
| law of mass action | The relationship of the amounts of reactants and products at equilibrium | The Equilibrium Constant |
| Le Chatelier’s principle | If an equilibrium is stressed, then the reaction shifts to reduce the stress | Shifting Equilibria: Le Chatelier’s Principle |
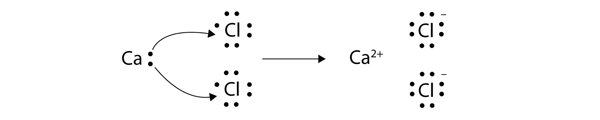
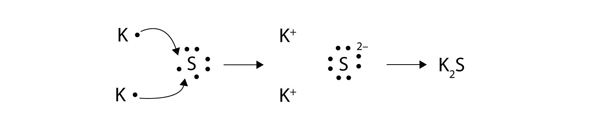
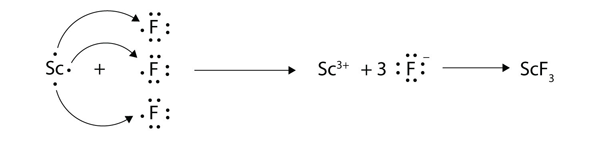
| Lewis diagram | A representation of the valence electrons of an atom that uses dots around the symbol of the element | Lewis Electron Dot Diagrams |
| limiting reagent | The reactant that runs out first for a given chemical reaction | Limiting Reagents |
| line spectrum | An image that contains only certain colors of light | Quantum Numbers for Electrons |
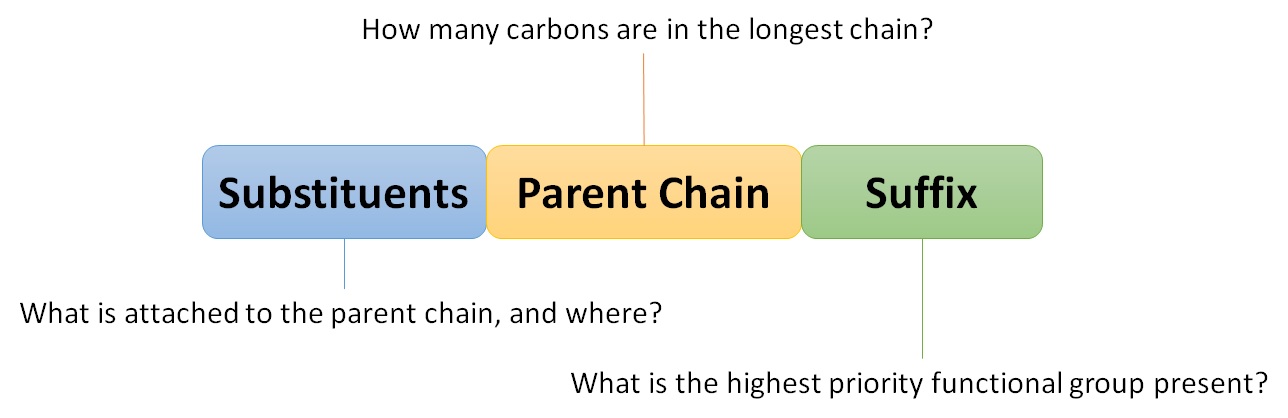
| locant | The numerical position of a substituent | Branched Hydrocarbons |
| lock and key model | A simple model used to describe enzyme activity, where substrates must fit into appropriately shaped active sites | Catalysis |
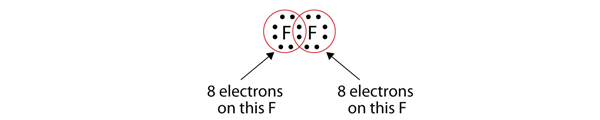
| lone electron pairs | A pair of electrons that does not make a covalent bond | Covalent Bonds |
| LUMO | The lowest unoccupied molecular orbital | Molecular Orbitals |
| magnetic quantum number (ml) | The index that determines the orientation of the electron’s spatial distribution | Quantum Numbers for Electrons |
| mass-mass calculation | A calculation in which you start with a given mass of a substance and calculate the mass of another substance involved in the chemical equation | Mole-Mass and Mass-Mass Calculations |
| matter | Anything that has mass and takes up space. is anything that has mass and takes up space | Some Basic Definitions |
| mean free path | The average distance traveled by a molecule between collisions | Molecular Effusion and Diffusion |
| melting | The process of a solid becoming a liquid | Phase Transitions: Melting, Boiling and Subliming |
| melting point | The characteristic temperature at which a solid becomes a liquid | Phase Transitions: Melting, Boiling and Subliming |
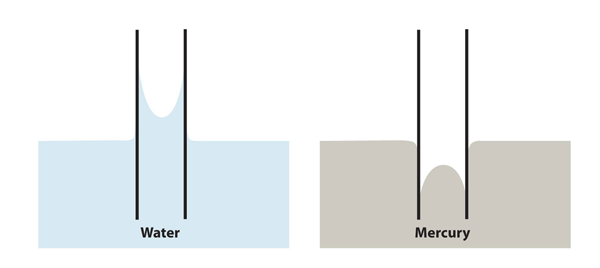
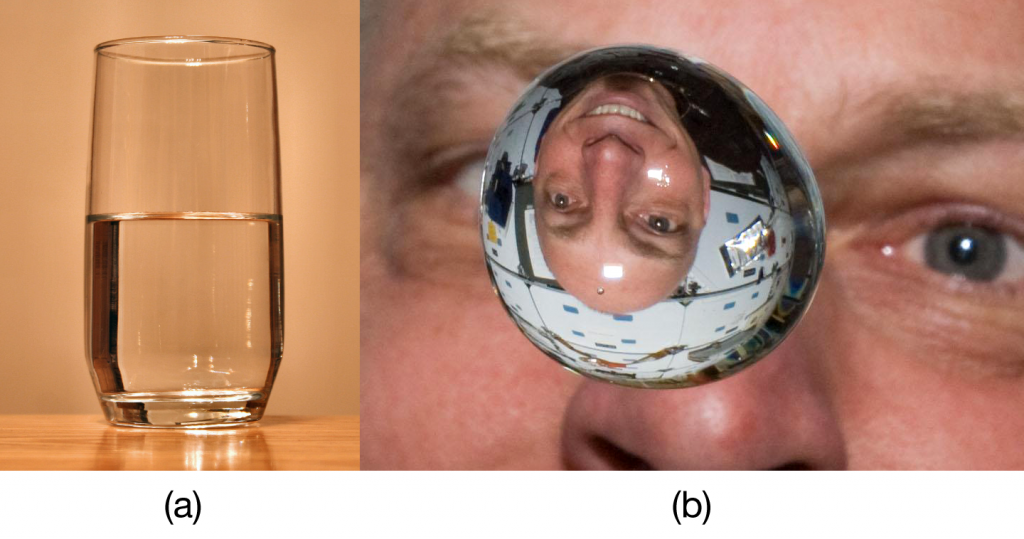
| meniscus | The curved surface a liquid makes as it approaches a solid barrier | Properties of Liquids |
| metal | An element that conducts electricity and heat well and is shiny, silvery, solid, ductile, and malleable | Some Basic Definitions |
| metallic solid | A solid with the characteristic properties of a metal | Solids |
| microstate (W) | A term used to describe different possible arrangements of molecular position and kinetic energy, at a particular thermodynamic state | Entropy and the Second Law of Thermodynamics |
| millimeters of mercury (mmHg) | The amount of pressure exerted by a column of mercury exactly 1 mm high | Pressure |
| mixture | A physical combination of more than one substance | Some Basic Definitions |
| molality (m) | The number of moles of solute per kilogram of solvent | Quantitative Units of Concentration |
| molar mass | The mass of 1 mol of a substance in grams | The Mole |
| molar volume | The volume of exactly 1 mol of a gas; equal to 22.4 L at STP | The Ideal Gas Law and Some Applications |
| molarity (M) | The number of moles of solute divided by the number of liters of solution | Quantitative Units of Concentration |
| mole | The number of things equal to the number of atoms in exactly 12 g of carbon-12; equals 6.022×1023 things | The Mole |
| mole fraction | The ratio of the number of moles of a component in a mixture divided by the total number of moles in the sample | Gas Mixtures |
| mole fraction | The ratio of the number of moles of a component to the total number of moles in a system | Colligative Properties of Solutions |
| molecular formula | A formal listing of what and how many atoms are in a molecule | Molecules an Chemical Nomenclature |
| molecular geometry | how the atoms in a molecule are arranged | Molecular Shapes and Polarity |
| molecular mass | The sum of the masses of the atoms in a molecule | Masses of Atoms and Molecules |
| molecular orbital theory (MO theory) | A more sophisticated model of chemical bonding where new molecular orbitals are generated using a mathematical process called Linear Combination of Atomic Orbitals (LCAO) | Molecular Orbitals |
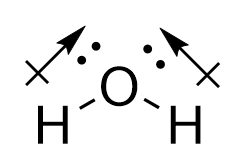
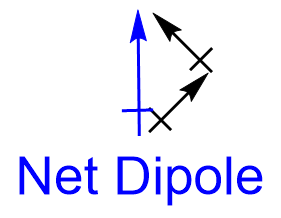
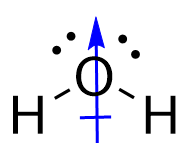
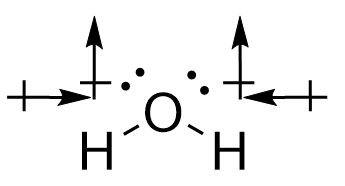
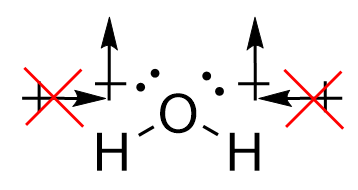
| molecular polarity | The vector sum of the individual bond dipoles | Molecular Shapes and Polarity |
| molecular solid | A crystalline solid whose components are covalently bonded molecules | Solids |
| molecularity | The total number of molecules that participate in the effective collision of the elementary step | Reaction Mechanisms |
| molecule | The smallest part of a substance that has the physical and chemical properties of that substance | Molecules an Chemical Nomenclature |
| mole-mass calculation | A calculation in which you start with a given number of moles of a substance and calculate the mass of another substance involved in the chemical equation, or vice versa | Mole-Mass and Mass-Mass Calculations |
| mole-mole calculation | A stoichiometry calculation when one starts with moles of one substance and convert to moles of another substance using the balanced chemical equation | The Mole in Chemical Reactions |
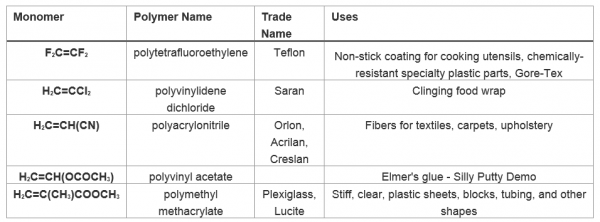
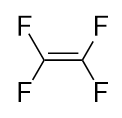
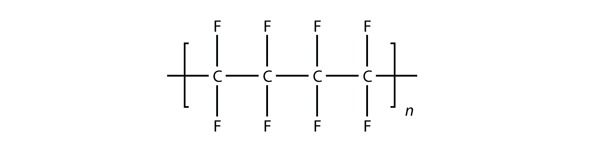
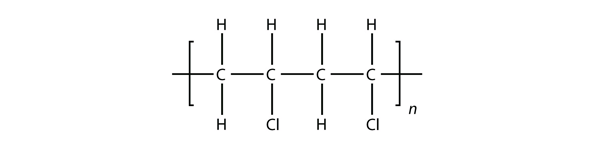
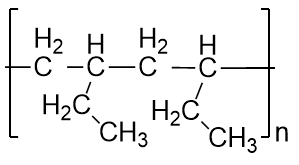
| monomer | The repeated unit of a polymer | Polymers |
| net ionic equation | A chemical equation with the spectator ions removed | Ionic Equations: A Closer Look |
| neutral salt | An ionic compound that does not affect the acidity of its aqueous solution | Strong and Weak Acids and Bases and Their Salts |
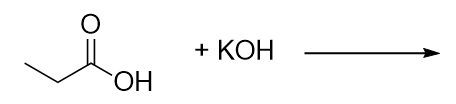
| neutralization reaction | The reaction of an acid with a base to produce water and a salt | Neutralization Reactions |
| neutralization reaction | The reaction of an acid and a base to produce water and a salt | Arrhenius Acids and Bases |
| neutron | A subatomic particle with no charge | Atomic Theory |
| node (nodal plane) | An area of zero electron density | Molecular Orbitals |
| nomenclature | The rules of naming in organic chemistry | Branched Hydrocarbons |
| nonmetal | An element that exists in various colors and phases, is brittle, and does not conduct electricity or heat well | Some Basic Definitions |
| nonpolar covalent bond | The equal sharing of electrons in a covalent bond | Other Aspects of Covalent Bonding |
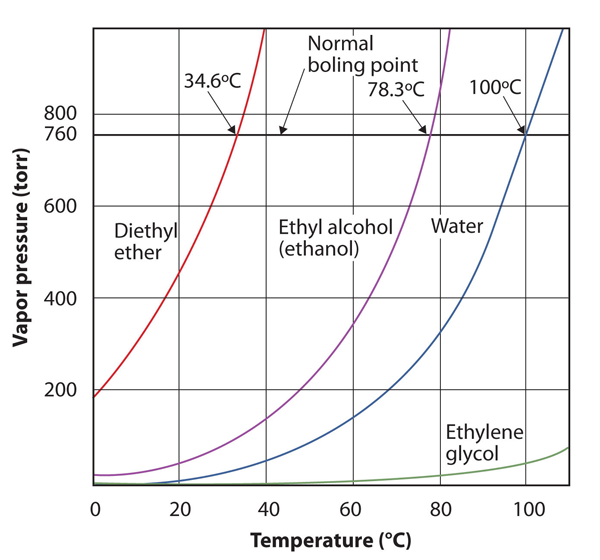
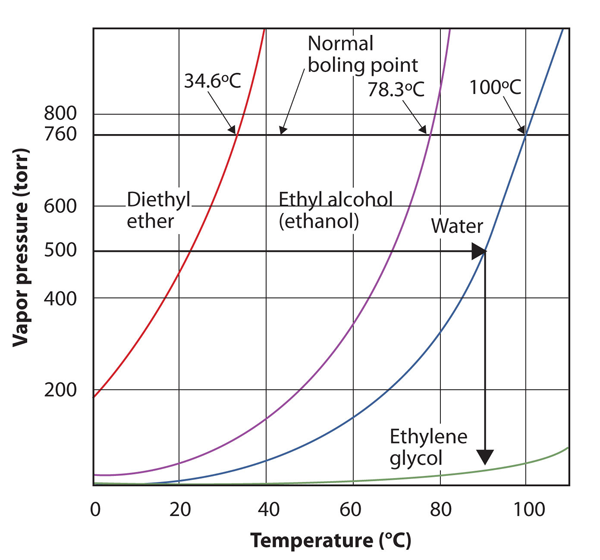
| normal boiling point | The characteristic temperature at which a liquid becomes a gas when the surrounding pressure is exactly 1 atm | Phase Transitions: Melting, Boiling and Subliming |
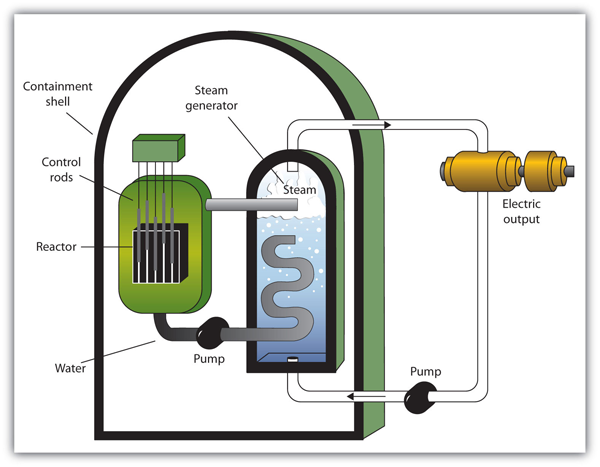
| nuclear energy | The controlled harvesting of energy from fission reactions | Nuclear Energy |
| nuclear equation | A chemical equation that emphasizes changes in atomic nuclei | Radioactivity |
| nuclear model | The model of an atom that has the protons and neutrons in a central nucleus with the electrons in orbit about the nucleus | Atomic Theory |
| nucleus | The center of an atom that contains protons and neutrons | Atomic Theory |
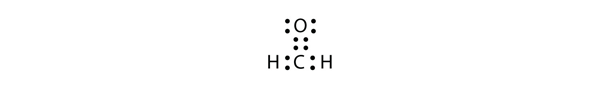
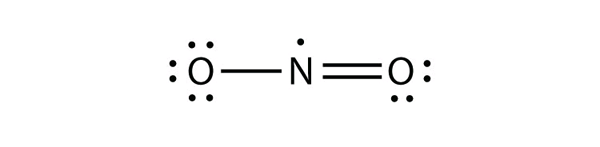
| odd-electron molecules | A molecule with an odd number of electrons in the valence shell of an atom | Violations of the Octet Rule |
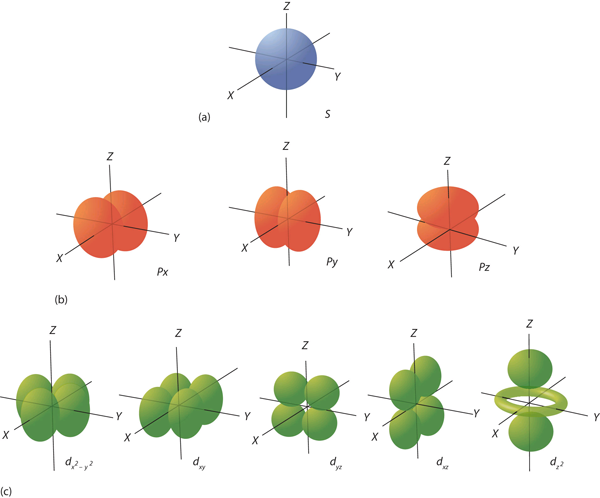
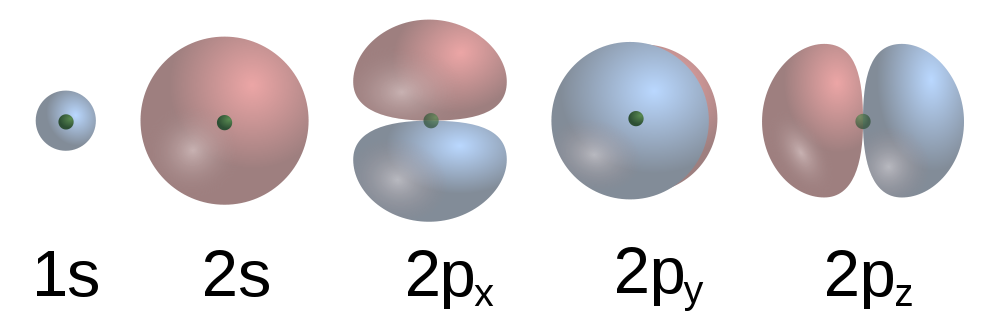
| orbital | The specific set of principal, angular momentum, and magnetic quantum numbers for an electron | Quantum Numbers for Electrons |
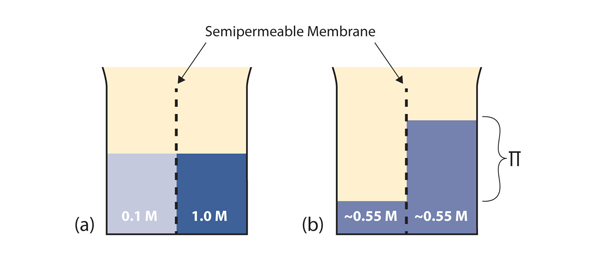
| osmosis | The tendency of solvent molecules to pass through a semipermeable membrane due to concentration differences | Colligative Properties of Solutions |
| osmotic pressure | The tendency of a solution to pass solvent through a semipermeable membrane due to concentration differences | Colligative Properties of Solutions |
| oxidation | The loss of one or more electrons by an atom; an increase in oxidation number | Oxidation-Reduction Reactions |
| oxidation | The loss of one or more electrons by an atom; an increase in oxidation number | Oxidation-Reduction Reactions |
| oxidation number | A number assigned to an atom that helps keep track of the number of electrons on the atom | Oxidation-Reduction Reactions |
| oxidation number | A number assigned to an atom that helps keep track of the number of electrons on the atom | Oxidation-Reduction Reactions |
| oxidation-reduction (redox) reactions | A chemical reaction that involves the transfer of electrons | Oxidation-Reduction Reactions |
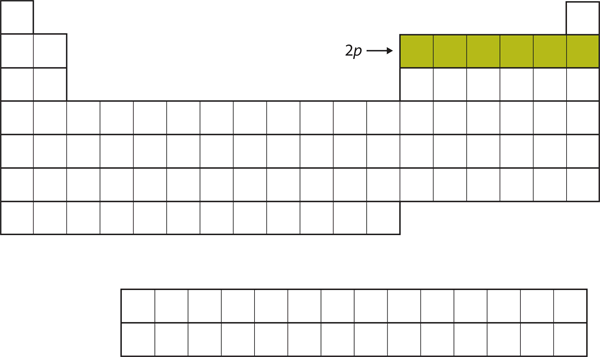
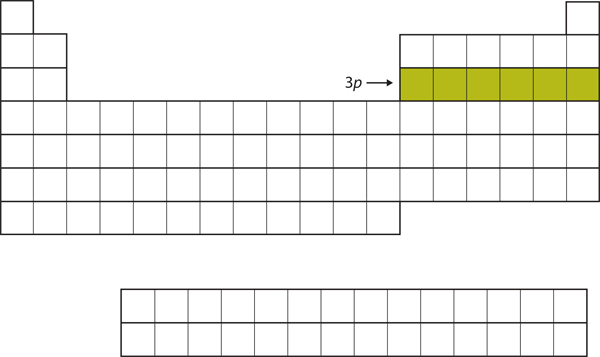
| p block | The columns of the periodic table in which p subshells are being occupied | Electronic Structure and the Periodic Table |
| parent isotope | The reactant in a nuclear equation | Radioactivity |
| parts per billion (ppb) | Ratio of mass of solute to total mass of sample times 1,000,000,000 | Quantitative Units of Concentration |
| parts per million (ppm) | Ratio of mass of solute to total mass of sample times 1,000,000 | Quantitative Units of Concentration |
| parts per thousand (ppth) | Ratio of mass of solute to total mass of sample times 1,000 | Quantitative Units of Concentration |
| Pauli exclusion principle | No two electrons in an atom can have the same set of four quantum numbers | Organization of Electrons in Atoms |
| percent yield | Actual yield divided by theoretical yield times 100% to give a percentage between 0% and 100% | Yields |
| percentage composition by mass (or mass percentage, % m/m) | Ratio of mass of solute to the total mass of a sample times 100 | Quantitative Units of Concentration |
| periodic table | A chart of all the elements | Atomic Theory |
| periodic trends | The variation of properties versus position on the periodic table | Periodic Trends |
| pH | The negative logarithm of the hydrogen ion concentration | The pH Scale |
| pH scale | The range of values from 0 to 14 that describes the acidity or basicity of a solution | The pH Scale |
| phase | An important physical property that defines whether matter is a solid, liquid, gas or supercritical fluid | Some Basic Definitions |
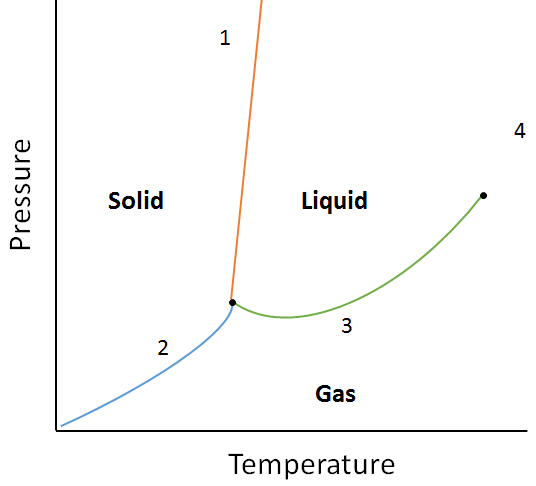
| phase diagram | A graphical representation of the equilibrium relationships that exist between the phases of a substance under specified pressures and temperatures | Properties of Liquids |
| photon | The name of a discrete unit of light acting as a particle | Light |
| physical change | A change that occurs when a sample of matter changes one or more of its physical properties | Some Basic Definitions |
| physical property | A characteristic that describes matter as it exists | Some Basic Definitions |
| pi bond (π bond) | The sideways overlap of p orbitals, placing electron density on opposite sides of the inter-nuclear axis – a double or triple bond | Valence Bond Theory and Hybrid Orbitals |
| Planck’s constant | The proportionality constant between the frequency and the energy of light: 6.626 × 10−34 J·s | Light |
| pOH | The negative logarithm of the hydroxide ion concentration | The pH Scale |
| polar covalent bond | A covalent bond between different atoms that attract the shared electrons by different amounts and cause an imbalance of electron distribution | Other Aspects of Covalent Bonding |
| polarity | A measure of the unequal sharing of electrons which has resulted in a dipole moment | Other Aspects of Covalent Bonding |
| polyatomic ions | An ion that contains more than one atom | Ions and Ionic Compounds |
| polymer | A long molecule made of many repeating units | Polymers |
| polymerization | The process of making a polymer | Polymers |
| polyprotic acid | An acid capable of donating more than one H+ ion | Some Special Types of Equilibria |
| precipitate | A solid that falls out of solution in a precipitation reaction | Types of Chemical Reactions: Single- and Double-Displacement Reactions |
| precipitation reaction | A chemical reaction in which two ionic compounds are dissolved in water and form a new ionic compound that does not dissolve | Types of Chemical Reactions: Single- and Double-Displacement Reactions |
| prefix | A prefix used with a unit that refers to a multiple or fraction of a fundamental unit to make a more conveniently sized unit for a specific quantity | Expressing Units |
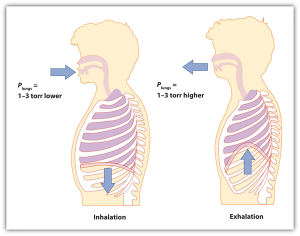
| pressure | Force per unit area | Pressure |
| primary battery | A battery that cannot be recharged | Applications of Redox Reactions: Voltaic Cells |
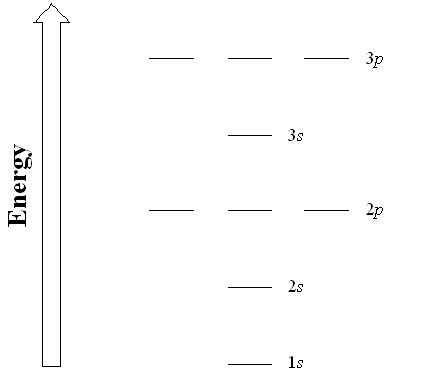
| principal quantum number (n) | The index that largely determines the energy of an electron in an atom | Quantum Numbers for Electrons |
| product | A final substance in a chemical equation | The Chemical Equation |
| proton | A subatomic particle with a positive charge | Atomic Theory |
| qualitative | A description of the quality of an object | Chemistry as a Science |
| quantitative | A description of a specific amount of something | Chemistry as a Science |
| quantization | When a quantity is restricted to having only certain values | Quantum Numbers for Electrons |
| quantum mechanics | The theory of electrons that treats them as a wave | Quantum Numbers for Electrons |
| quantum number | An index that corresponds to a property of an electron, like its energy | Quantum Numbers for Electrons |
| rad | A unit of radioactive exposure equal to 0.01 J/g of tissue | Units of Radioactivity |
| radioactive decay | The spontaneous change of a nucleus from one element to another | Radioactivity |
| radioactivity | Emanations of particles and radiation from atomic nuclei | Radioactivity |
| Raoult’s law | The mathematical formula for calculating the vapor pressure of a solution | Colligative Properties of Solutions |
| rate constant (k) | A proportionality constant specific to each reaction at a particular temperature | Rate Laws |
| rate-determining step | The slowest step in a multistep mechanism | Reaction Mechanisms |
| rate law | A mathematical relationship between the reaction rate and the reactant concentrations | Rate Laws |
| reactant | An initial substance in a chemical equation | The Chemical Equation |
| reaction mechanism | The bond making and bond breaking steps which occur at the molecular level during a chemical reaction | Reaction Mechanisms |
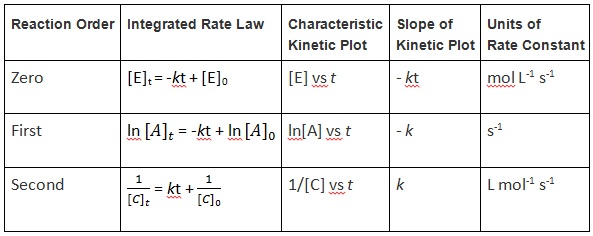
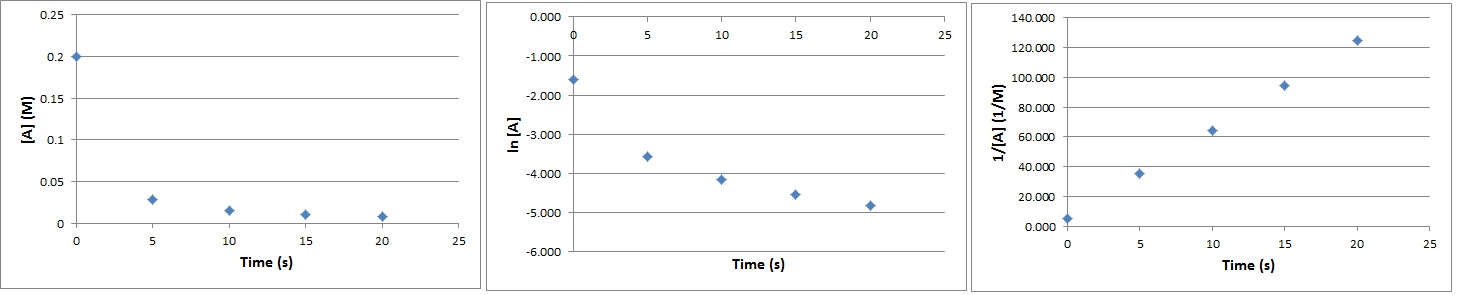
| reaction order | The sum of the concentration term exponents in a rate law equation | Rate Laws |
| reaction rate | The speed of a chemical reaction | Introduction to Kinetics |
| real gases | A gas that deviates from ideal behaviour | Real Gases |
| redox reaction | A chemical reaction that involves the transfer of electrons | Oxidation-Reduction Reactions |
| reduction | The gain of one or more electrons by an atom; a decrease in oxidation number | Oxidation-Reduction Reactions |
| reduction | The gain of one or more electrons by an atom; a decrease in oxidation number | Oxidation-Reduction Reactions |
| rem | A unit of radioactive exposure that includes a factor to account for the type of radioactivity | Units of Radioactivity |
| ribozyme | Ribonucleic acid (RNA) molecules capable of catalyzing certain chemical reactions | Catalysis |
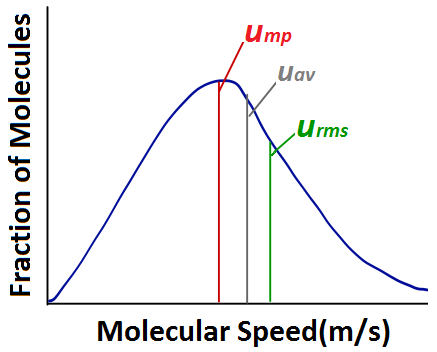
| root-mean-square (rms) speed (urms) | The speed of molecules having exactly the same kinetic energy as the average kinetic energy of the sample | Kinetic-Molecular Theory of Gases |
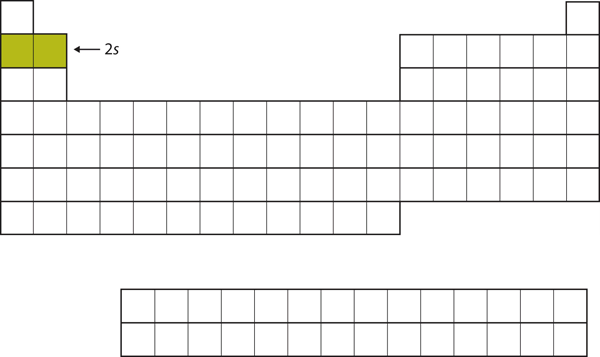
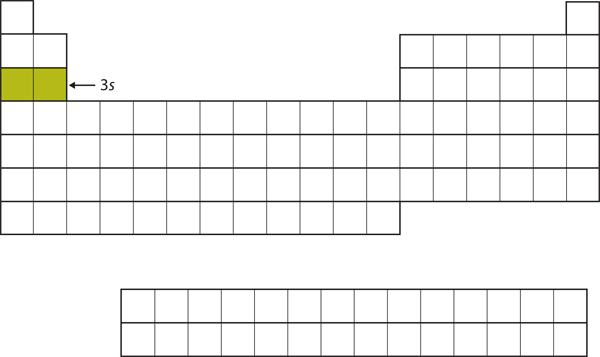
| s block | The columns of the periodic table in which s subshells are being occupied | Electronic Structure and the Periodic Table |
| salt | Any ionic compound that is formed from a reaction between an acid and a base | Neutralization Reactions |
| salt | Any ionic compound that is formed from a reaction between an acid and a base | Arrhenius Acids and Bases |
| salt bridge | A part of a voltaic cell that contains a solution of some ionic compound whose ions migrate to either side of the voltaic cell to maintain the charge balance | Applications of Redox Reactions: Voltaic Cells |
| saturated hydrocarbons | A carbon compound with the maximum possible number of H atoms in its formula | Hydrocarbons |
| saturated solution | A solution with the maximum amount of solute dissolved in it | Some Definitions |
| science | The process of knowing about the natural universe through observation and experiment | Chemistry as a Science |
| scientific law | A specific statement that is thought to be never violated by the entire natural universe | Chemistry as a Science |
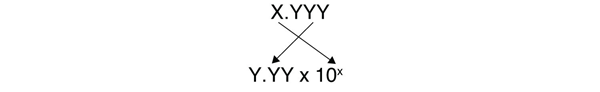
| scientific notation | An expression of a number using powers of 10 | Expressing Numbers |
| screening | The repelling valence electrons by core electrons | Periodic Trends |
| second law of thermodynamics | A spontaneous process will increase the entropy of the universe | Entropy and the Second Law of Thermodynamics |
| secondary battery | A battery that can be recharged | Applications of Redox Reactions: Voltaic Cells |
| semimetal | An element that has properties of both metals and nonmetals | Some Basic Definitions |
| semipermeable membrane | A thin membrane that will pass certain small molecules but not others | Colligative Properties of Solutions |
| SI unit | International System of Units used by all scientists, literally translated from “le Système International d’unités.” | Expressing Units |
| Sievert (Sv) | Sievert (Sv) is a related unit and is defined as 100 rem | Units of Radioactivity |
| sigma bond (σ bond) | Orbital overlap to form a bond which has cylindrical symmetry – a single bond | Valence Bond Theory and Hybrid Orbitals |
| significant figures | The limit of the number of places a measurement can be properly expressed with | Significant Figures |
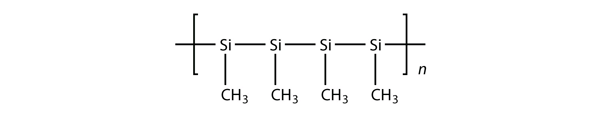
| silicones | A polymer based on a silicon and oxygen backbone | Polymers |
| single bond | A covalent bond composed of one pair of electrons | Covalent Bonds |
| single-replacement reaction | A chemical reaction in which one element is substituted for another element in a compound | Types of Chemical Reactions: Single- and Double-Displacement Reactions |
| solidification | The process of a liquid becoming a solid | Phase Transitions: Melting, Boiling and Subliming |
| solubility | The maximum amount of a solute that can be dissolved in a given amount of a solvent | Some Definitions |
| solubility rules | General statements that predict which ionic compounds dissolve and which do not | Types of Chemical Reactions: Single- and Double-Displacement Reactions |
| solute | The minor component of a solution | Some Definitions |
| solution | See homogeneous mixture | Some Basic Definitions |
| solvent | The major component of a solution | Some Definitions |
| specific heat capacity | The proportionality constant between heat, mass, and temperature change; also called specific heat | Work and Heat |
| spectator ion | An ion that does nothing in the overall course of a chemical reaction | Ionic Equations: A Closer Look |
| spin quantum number (ms) | The index that indicates one of two spin states for an electron | Quantum Numbers for Electrons |
| spontaneous process | A process that occurs without the influence of external forces or a change that moves a system towards equilibrium | Spontaneous Change |
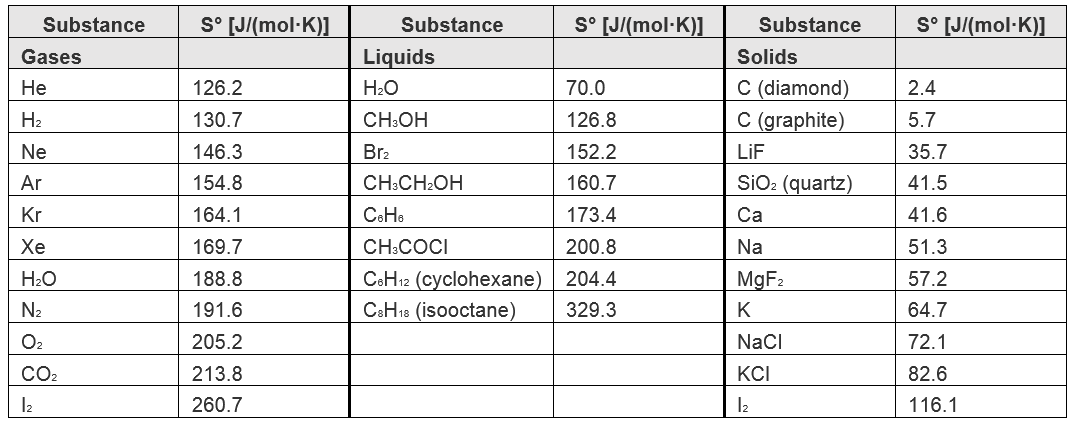
| standard molar entropy (So) | The entropy of 1 mole of a substance in its standard state, at 1 atm of pressure | Measuring Entropy and Entropy Changes |
| standard notation | A straightforward expression of a number | Expressing Numbers |
| standard temperature and pressure (STP) | A set of benchmark conditions used to compare other properties of gases; 100 kPa for pressure and 273 K for temperature | The Ideal Gas Law and Some Applications |
| stoichiometry | The relating of one chemical substance to another using a balanced chemical reaction | Stoichiometry |
| strong acid | Any acid that is 100% dissociated into ions in aqueous solution | Strong and Weak Acids and Bases and Their Salts |
| strong base | Any base that is 100% dissociated into ions in aqueous solution | Strong and Weak Acids and Bases and Their Salts |
| sublimation | The process of a solid becoming a gas | Phase Transitions: Melting, Boiling and Subliming |
| subshell | A term used to describe electrons in a shell that have the same angular momentum quantum number | Quantum Numbers for Electrons |
| substance | Matter that has the same physical and chemical properties throughout. | Some Basic Definitions |
| substituent | A branch off a main chain in a hydrocarbon | Branched Hydrocarbons |
| substrate | The reactants which are specific for a biological catalyst | Catalysis |
| supercritical fluid | A phase beyond the critical point, where liquid and gas phases are no longer distinct | Properties of Liquids |
| supersaturated solution | A unstable solution with more than the normal maximum amount of solute in it | Some Definitions |
| surface tension | An effect caused by an imbalance of forces on the atoms at the surface of a liquid | Properties of Liquids |
| surrounding atoms | An atom that makes covalent bonds to the central atom(s) | Covalent Bonds |
| system | The part of the universe under study | Energy |
| temperature | A measure of the average amount of kinetic energy a system contains | Other Units: Temperature and Density |
| theoretical yield | An amount that is theoretically produced as calculated using the balanced chemical reaction | Yields |
| theory | A general statement that explains a large number of observations | Chemistry as a Science |
| thermochemical equation | A chemical equation that includes an enthalpy change | Enthalpy and Chemical Reactions |
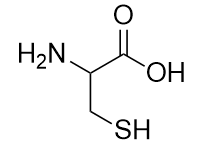
| thiol | The sulfur analog of an alcohol | Other Functional Groups |
| third law of thermodynamics | At absolute zero the entropy of a pure, perfect crystal is zero | Measuring Entropy and Entropy Changes |
| titrant | The reagent of known concentration | Acid-Base Titrations |
| titration | A chemical reaction performed quantitatively to determine the exact amount of a reagent | Acid-Base Titrations |
| torr | Another name for a millimeter of mercury | Pressure |
| tracer | A substance that can be used to follow the pathway of that substance through a structure | Uses of Radioactive Isotopes |
| transition state | The highest energy transitional point in the elementary step | Reaction Mechanisms |
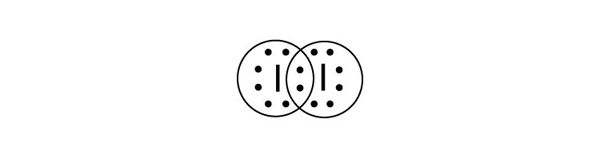
| triple bond | A covalent bond composed of three pairs of bonding electrons | Covalent Bonds |
| unsaturated hydrocarbons | A carbon compound with less than the maximum possible number of H atoms in its formula | Hydrocarbons |
| unsaturated solution | A solution with less than the maximum amount of solute dissolved in it | Some Definitions |
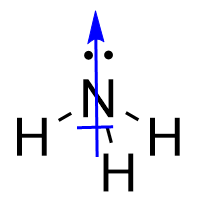
| valence electrons | The electrons in the highest-numbered shell, plus any electrons in the last unfilled subshell. The electrons most likely involved in chemical reactions | Electronic Structure and the Periodic Table |
| valence shell | The highest-numbered shell in an atom that contains electrons | Electronic Structure and the Periodic Table |
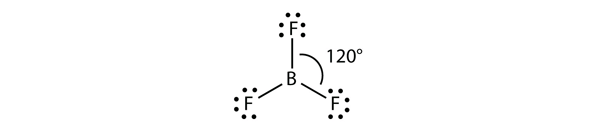
| valence shell electron pair repulsion theory (VSEPR) | The general concept that estimates the shape of a simple molecule: electron pairs repel each other to get as far away from each other as possible | Molecular Shapes and Polarity |
| van der Waal’s equation | An equation which compensates for deviations from ideal gas behaviour, correcting for intermolecular forces and the volume of gas molecules | Real Gases |
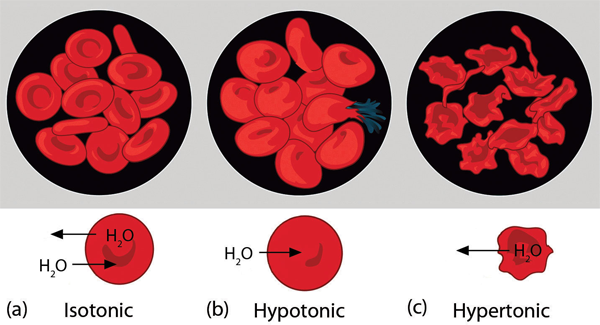
| van’t Hoff factor (i) | The number of particles each solute formula unit breaks apart into when it dissolves | Colligative Properties of Ionic Solutes |
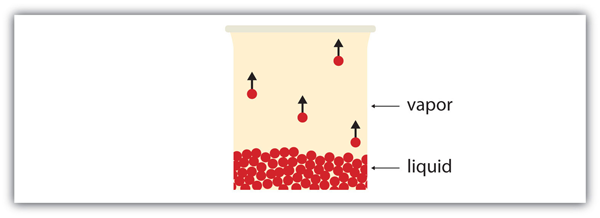
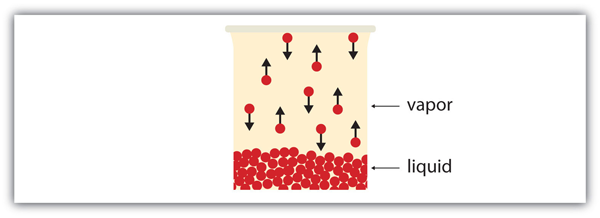
| vapor | Material in the gas phase due to evaporation | Properties of Liquids |
| vapor pressure | The partial pressure exerted by evaporation of a liquid | Gas Mixtures |
| vapor pressure depression | The decrease of a solution’s vapor pressure because of the presence of a solute | Colligative Properties of Solutions |
| vector quantity | A quantity which has both a magnitude and direction | Molecular Shapes and Polarity |
| voltaic (galvanic) cell | An apparatus that allows for useful electrical work to be extracted from a redox reaction. | Applications of Redox Reactions: Voltaic Cells |
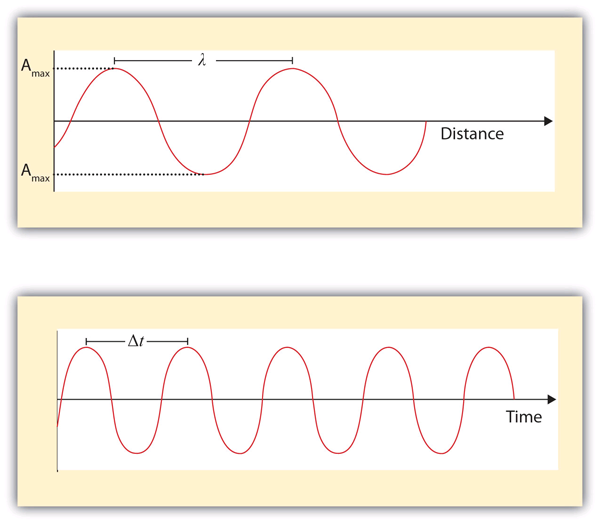
| wavelength | The distance between corresponding points in two adjacent light cycles | Light |
| weak acid | Any acid that is less than 100% dissociated into ions in aqueous solution | Strong and Weak Acids and Bases and Their Salts |
| weak base | Any base that is less than 100% dissociated into ions in aqueous solution | Strong and Weak Acids and Bases and Their Salts |














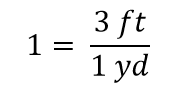
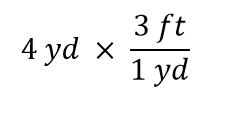
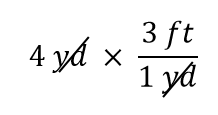
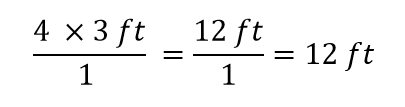
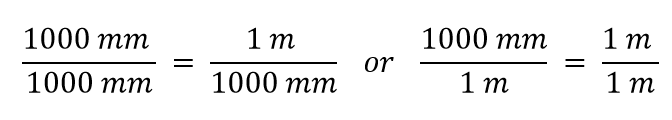
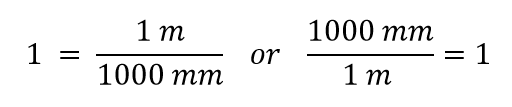
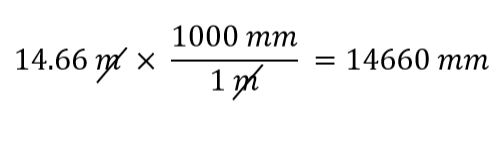
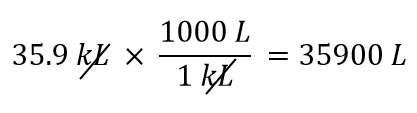

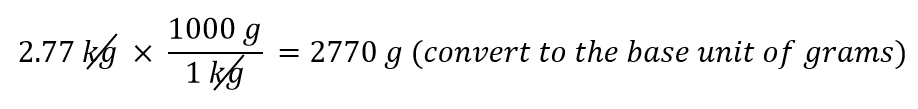
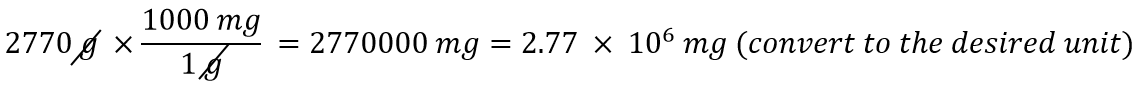
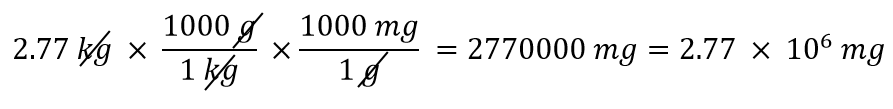
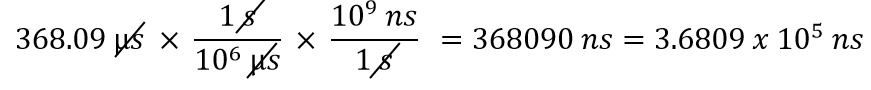
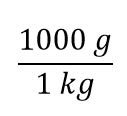
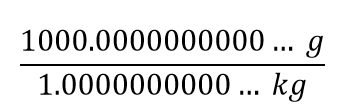
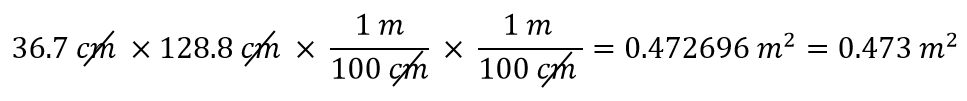


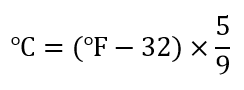
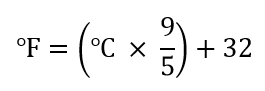
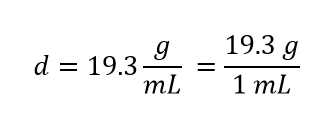
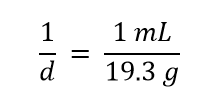
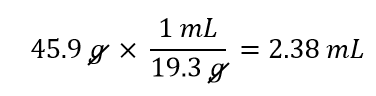
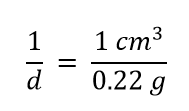
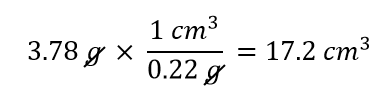


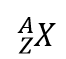
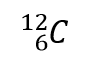
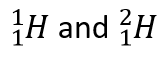




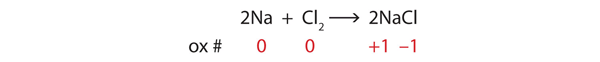
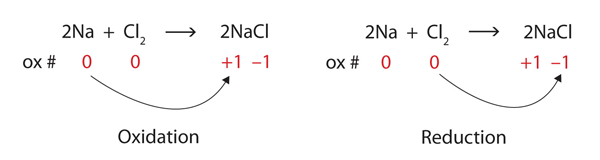
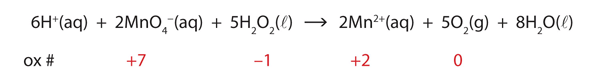


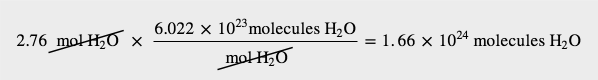
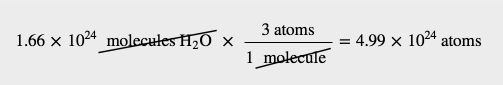
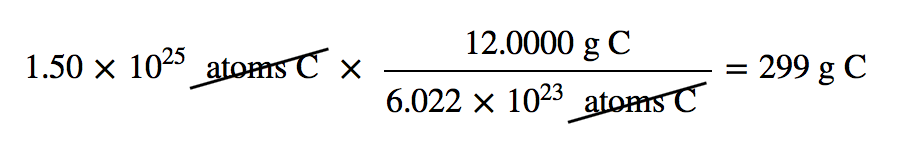
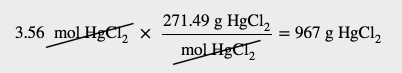
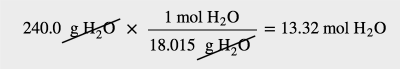
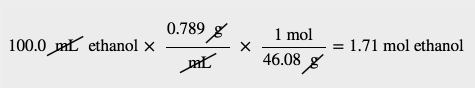
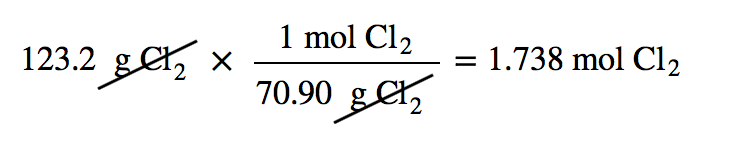

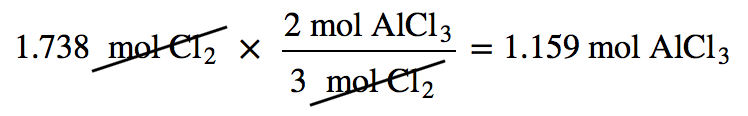

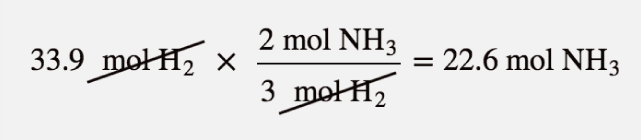
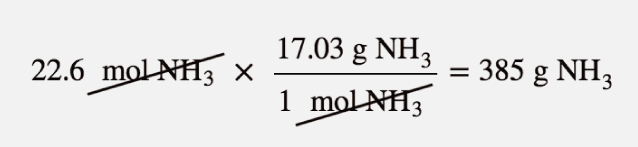
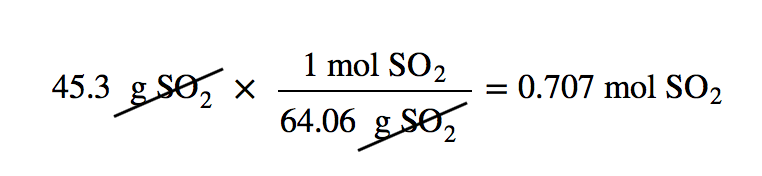
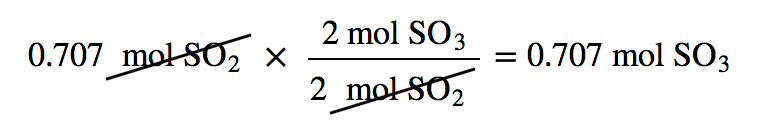
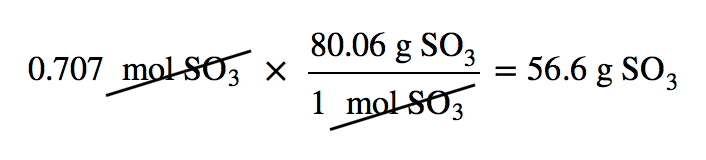
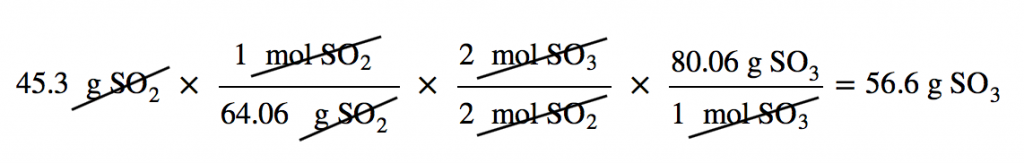
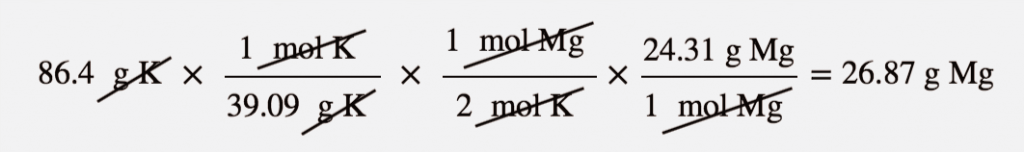
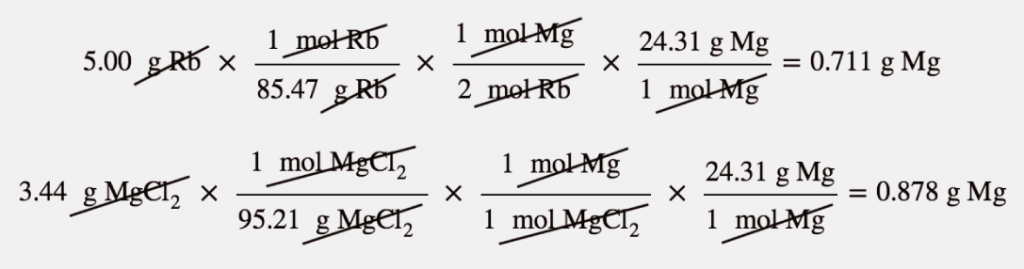
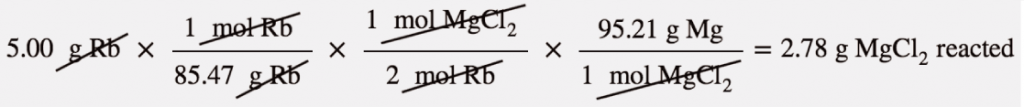
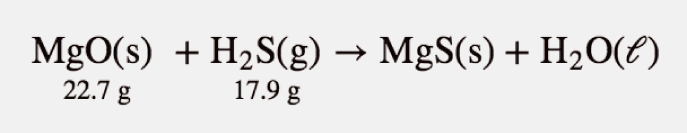
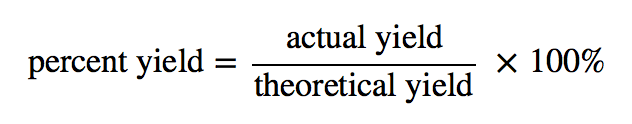
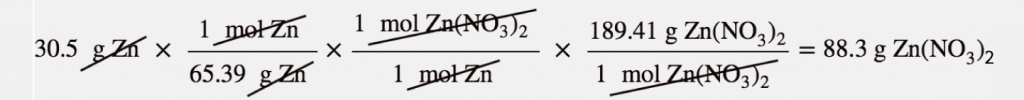
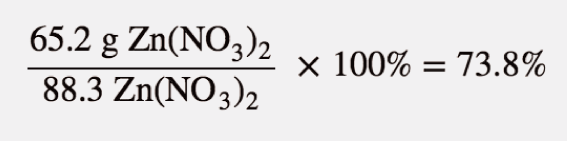
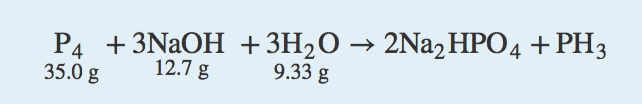

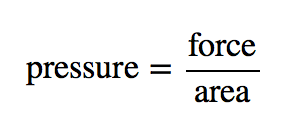

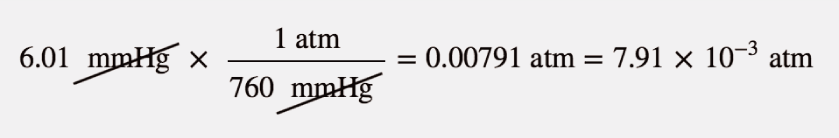
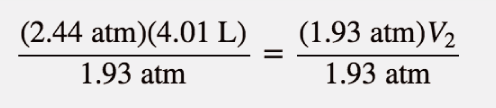
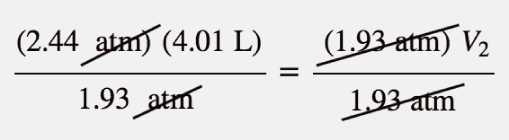

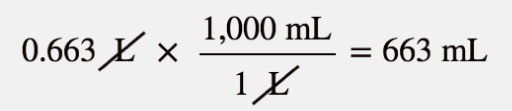
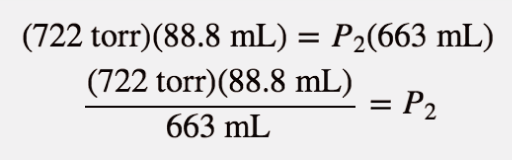
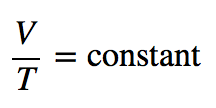

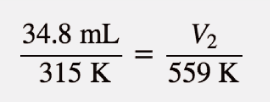
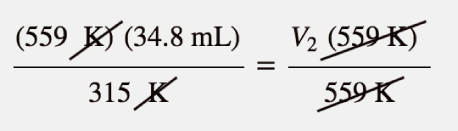
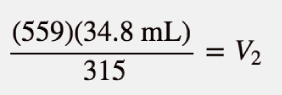
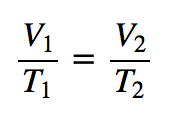
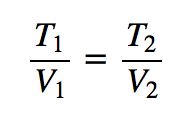
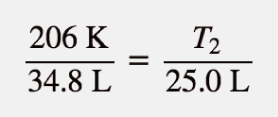
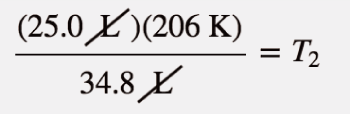


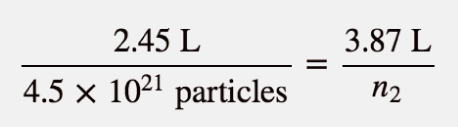
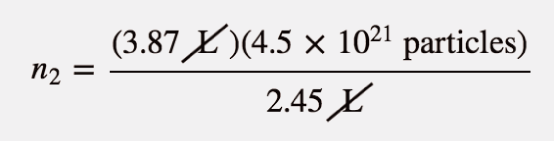
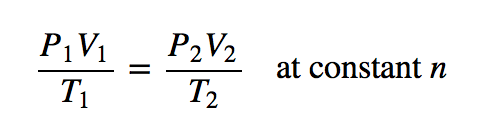
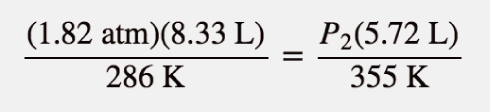
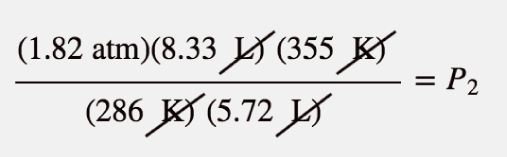
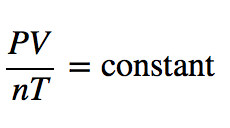
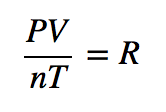
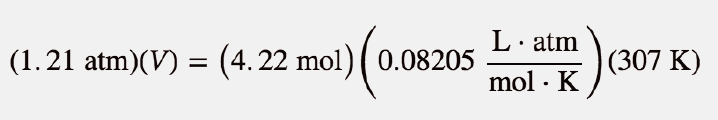
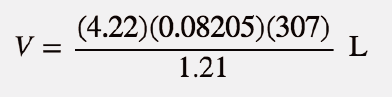
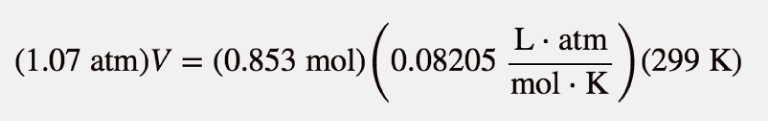
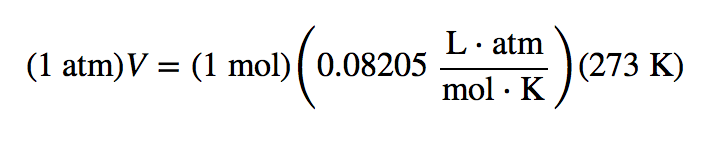
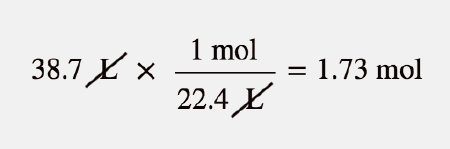
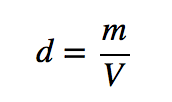
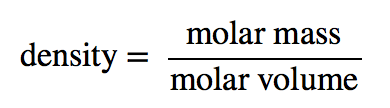
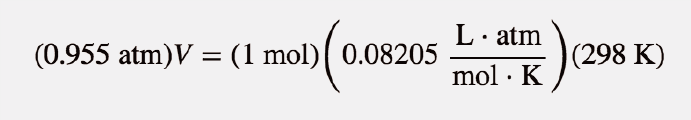
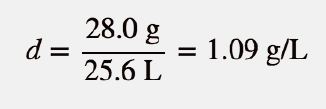
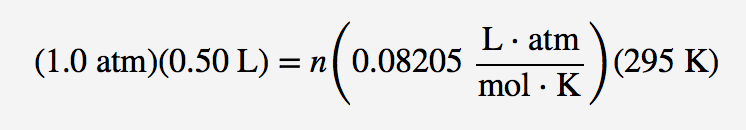
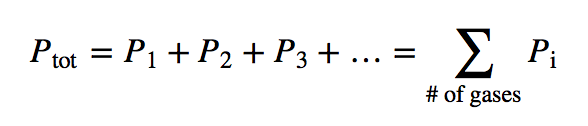
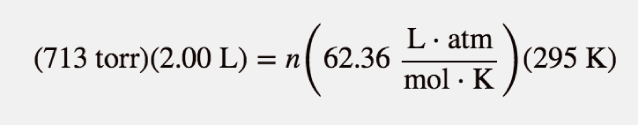
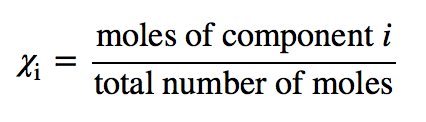
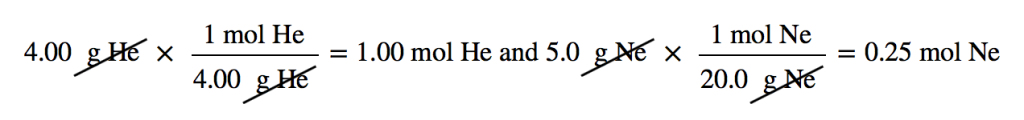
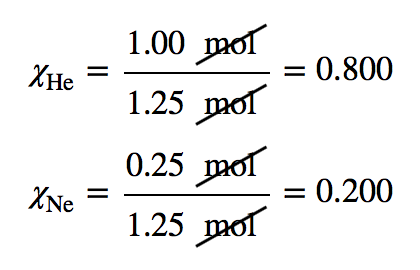
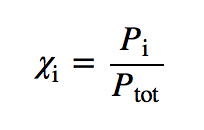




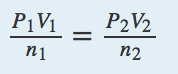

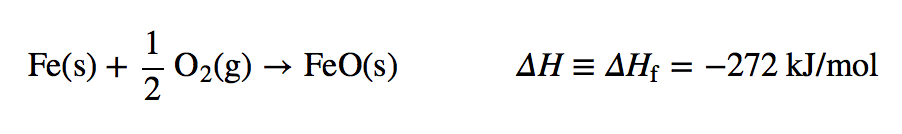
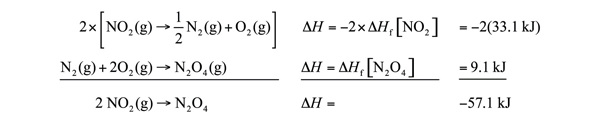
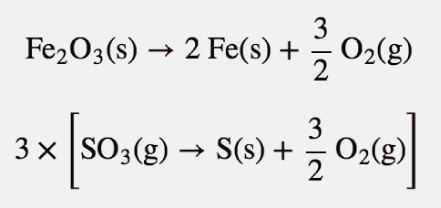
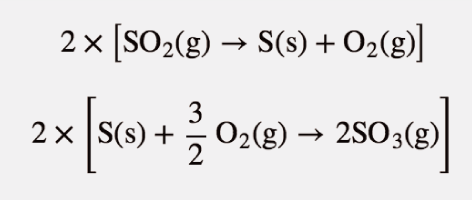
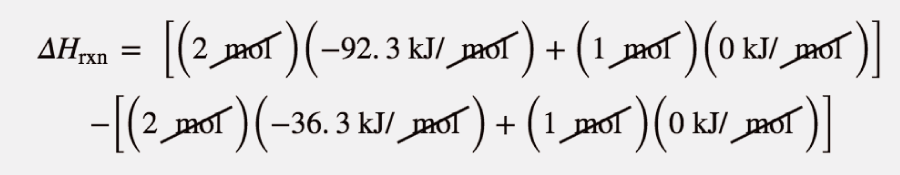
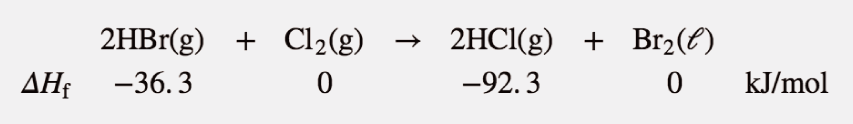
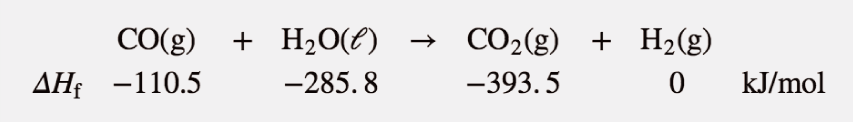
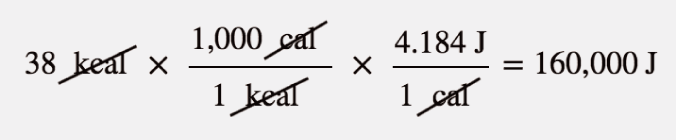
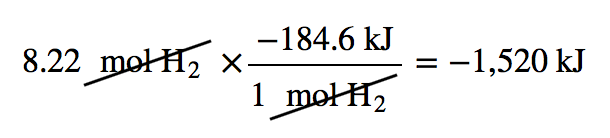
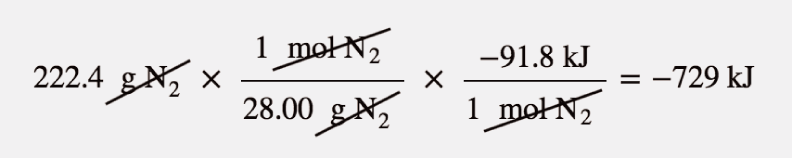
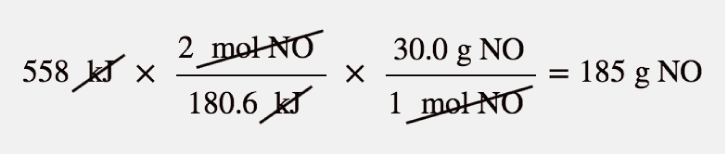

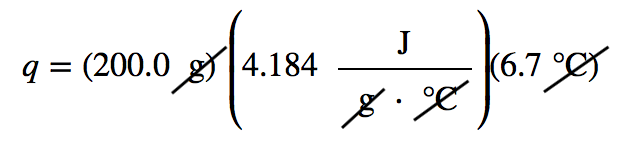
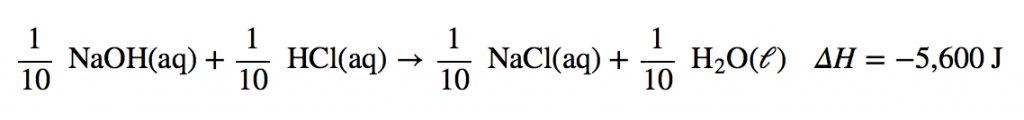
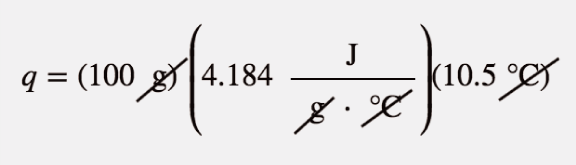
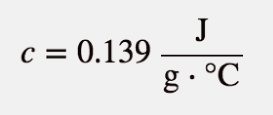
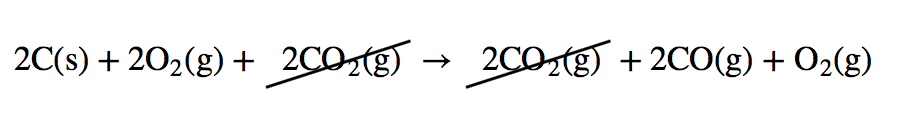
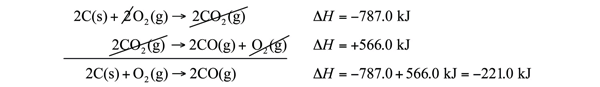
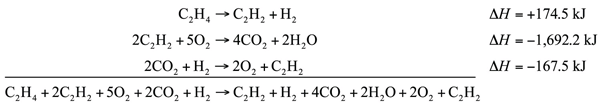

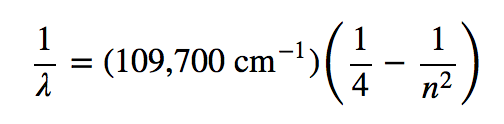





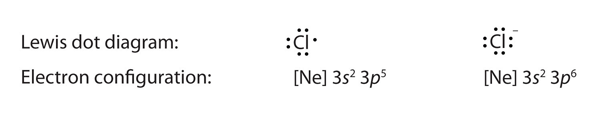
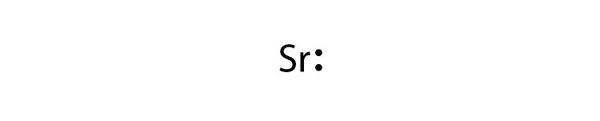

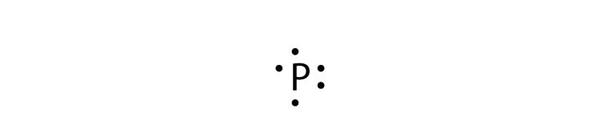
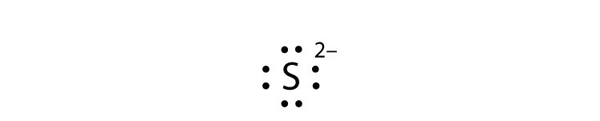
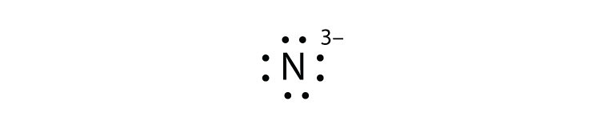

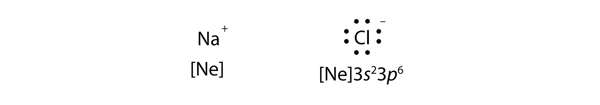
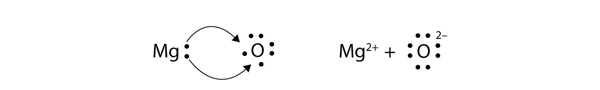
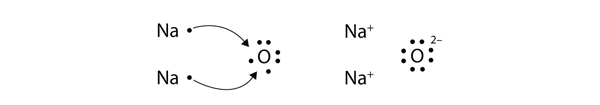




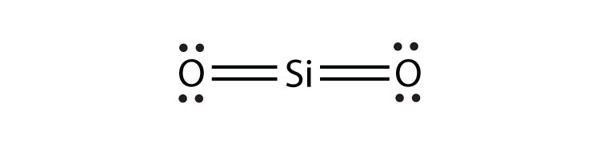
![Figure 9.3 Electronegativities by Elements. By Joanjoc at ca.wikipedia [Public domain], from Wikimedia Commons](http://opentextbc.ca/introductorychemistry/wp-content/uploads/sites/17/2014/09/Taula_periòdica_electronegativitat.png)
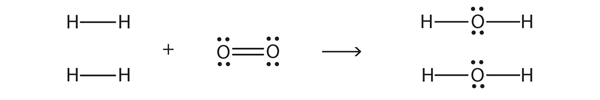
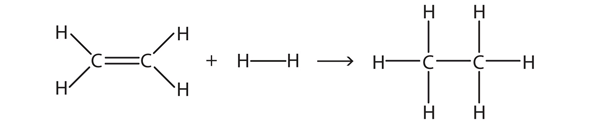
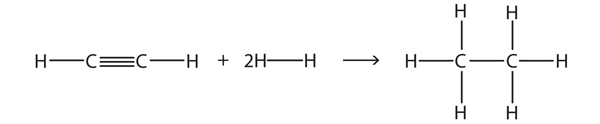

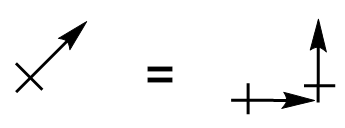
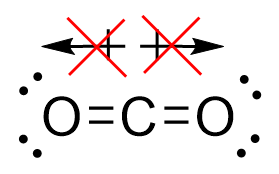





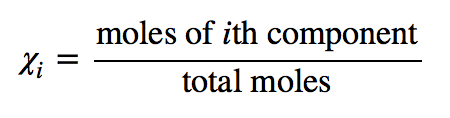
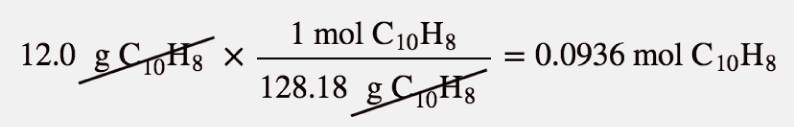
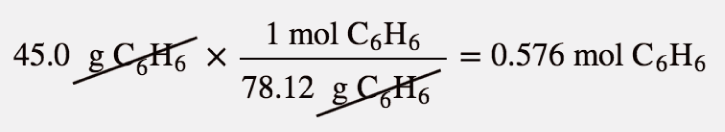

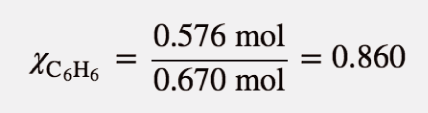
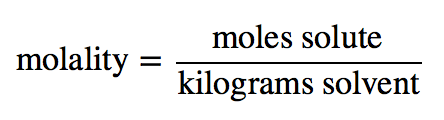

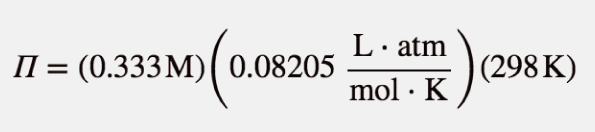
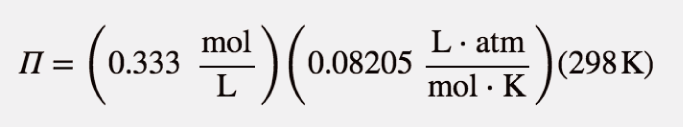

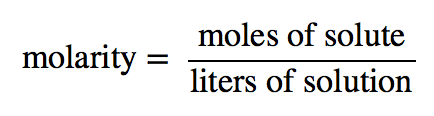
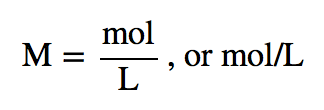
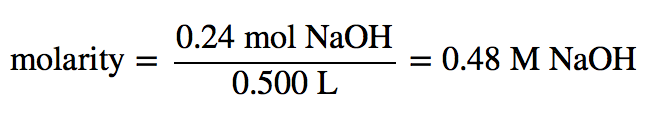
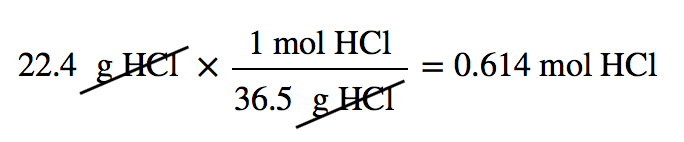
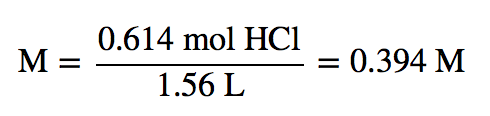
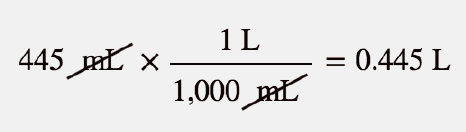
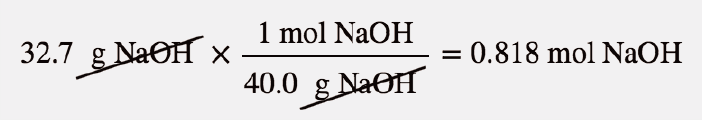
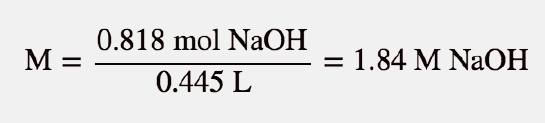
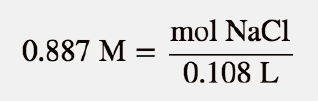
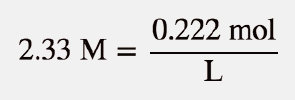
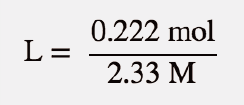
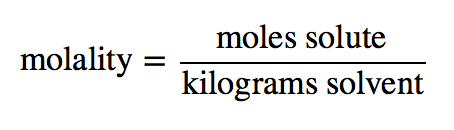
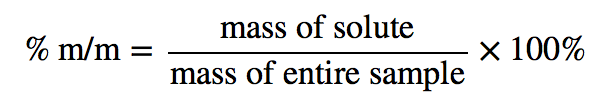
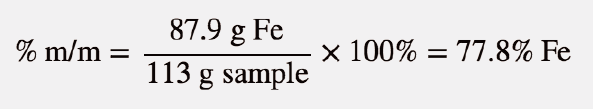
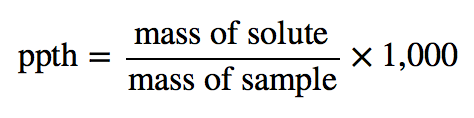
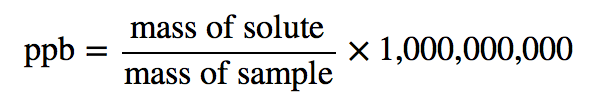
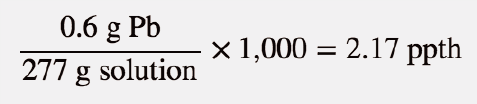
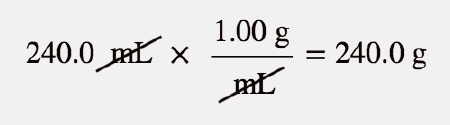
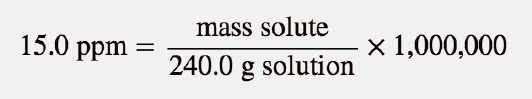
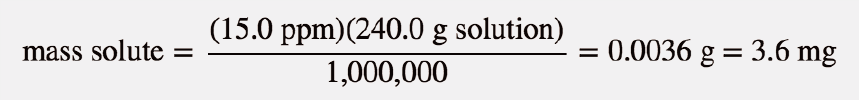


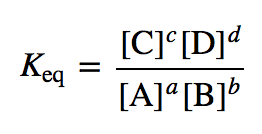
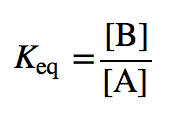
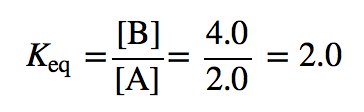
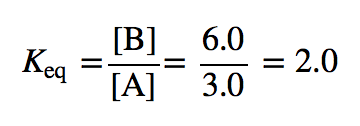
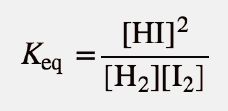
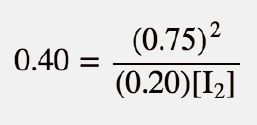
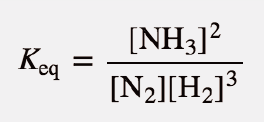
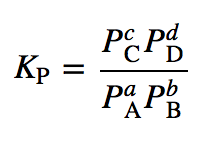
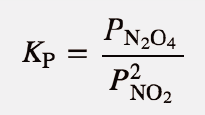
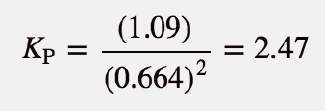
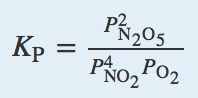

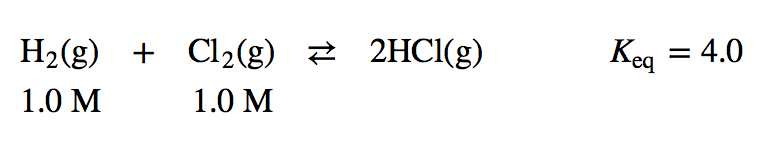
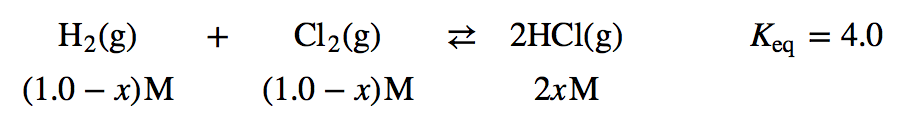
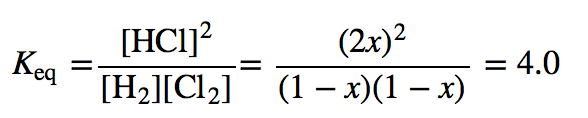
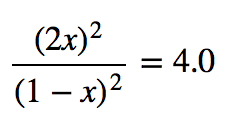
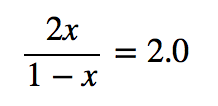
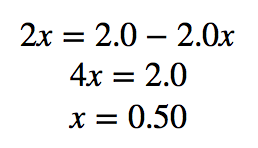
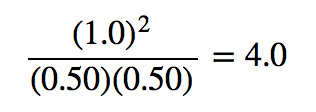
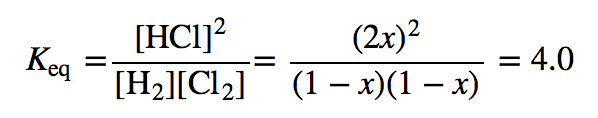
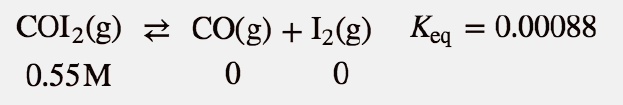
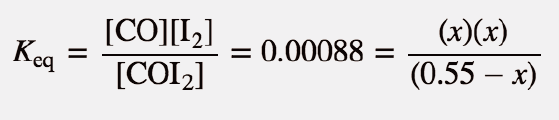
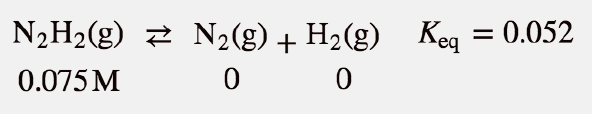
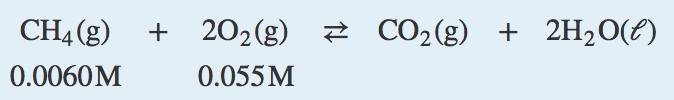
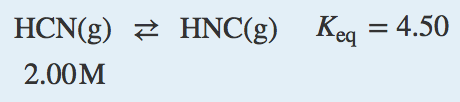
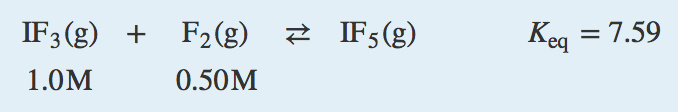





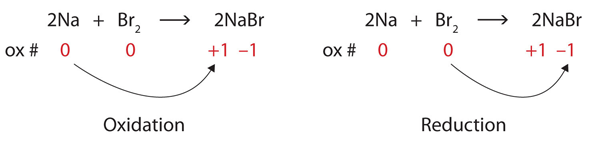
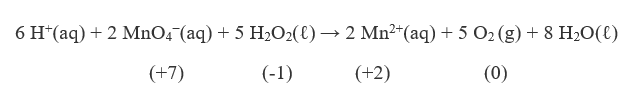




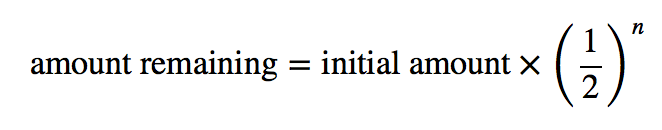

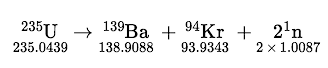
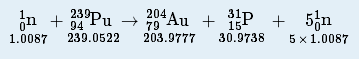



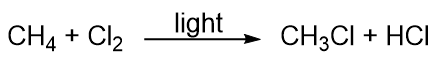

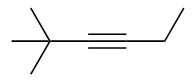

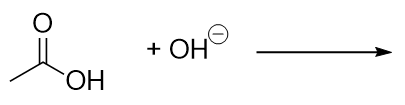
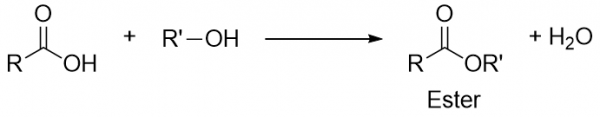
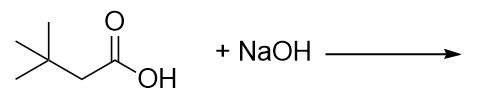
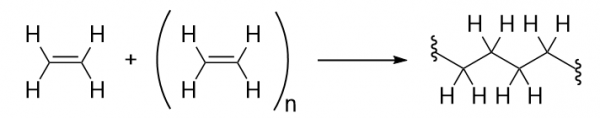



![Figure 17.4.2. Plot of 1/[C] versus time for a second-order reaction](http://opentextbc.ca/introductorychemistry/wp-content/uploads/sites/17/2016/01/Second-order-reaction-plot-1.jpg)