National Collaborating Centre for Methods and Tools. (n.d.). 6S Search Pyramid Tool [online video]. https://www.nccmt.ca/training/videos?v=125#ure4
Polit, D. F., & Beck, C. T. (2021). Essentials of nursing research: Appraising evidence for nursing practice (10th ed). Philadelphia, PA: Lippincott, Williams, & Wilkins.
Singh, M., Thirsk, L., Stahlke, S., Venkatesaperumal, R., LoBiondo-Wood, G., & Haber, J. (2022). Nursing research in Canada, methods, critical appraisal and utilization (5th ed.) Toronto, Canada: Elsevier. ISBN: 978-0-323-77898-5


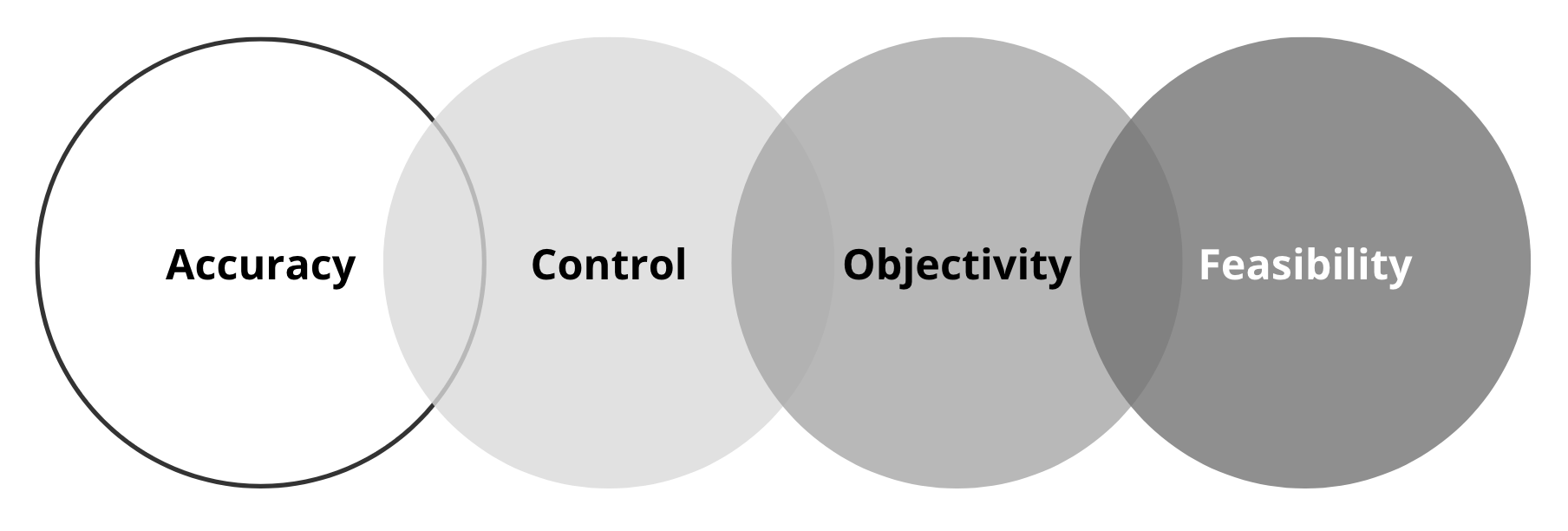
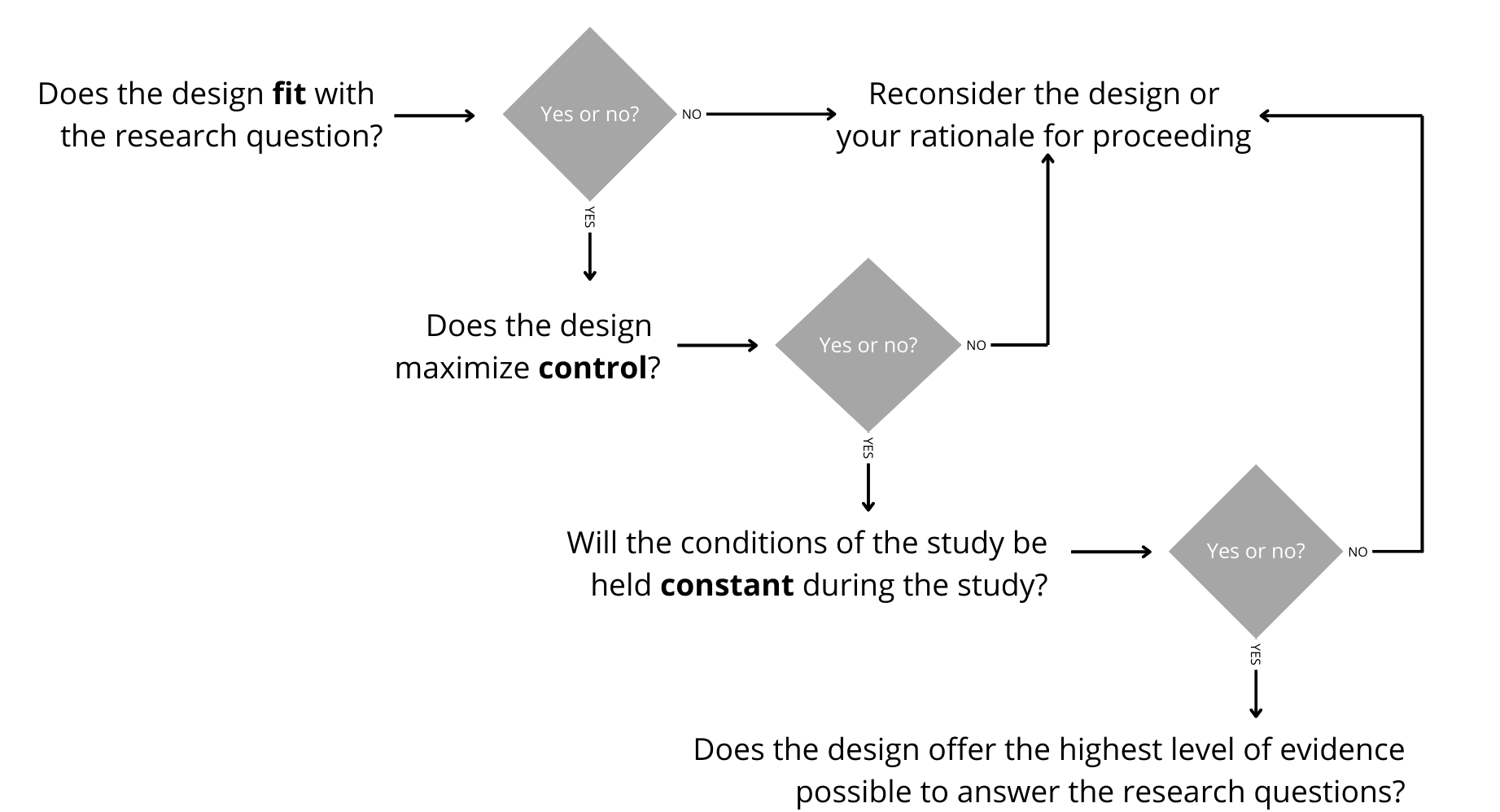
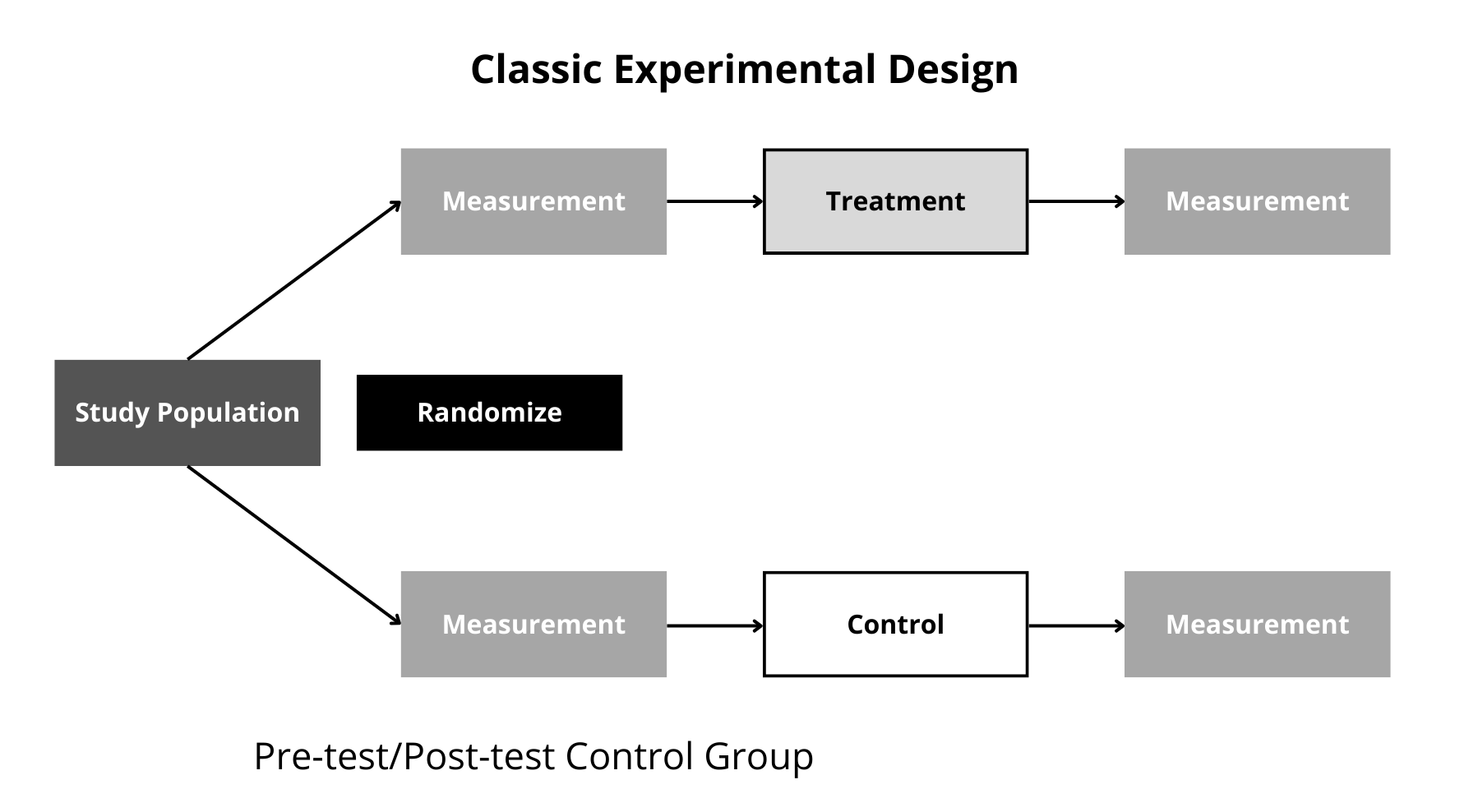
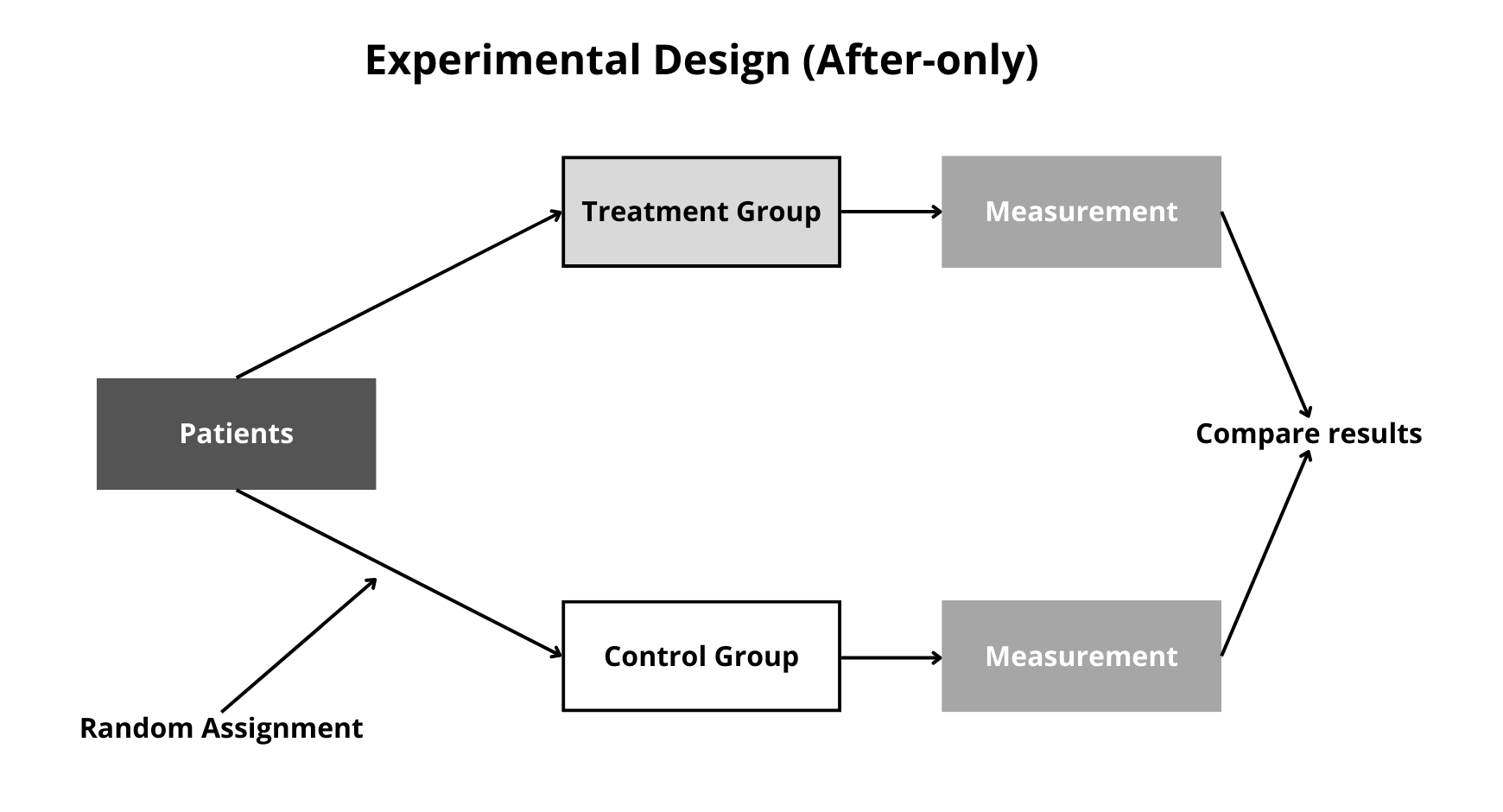
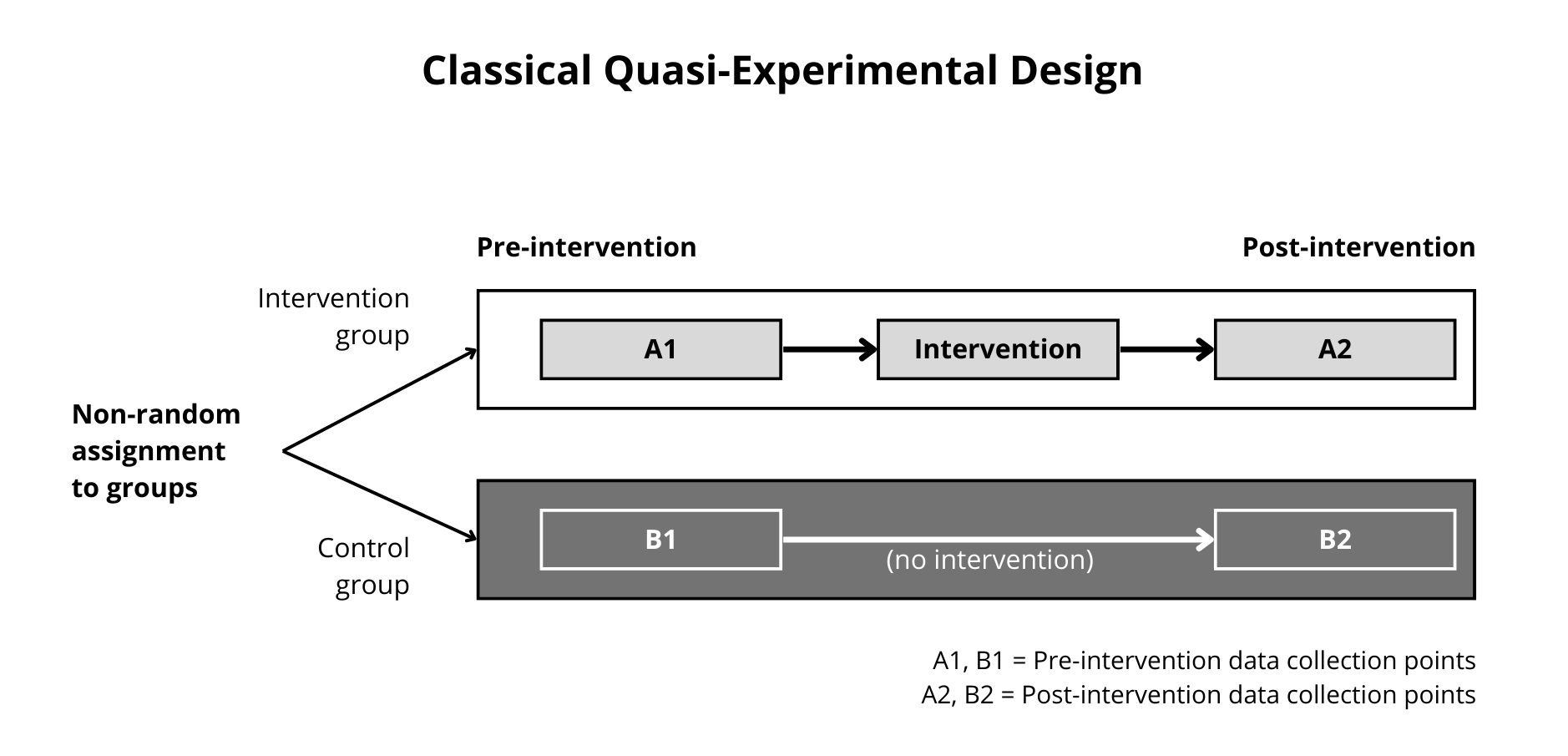
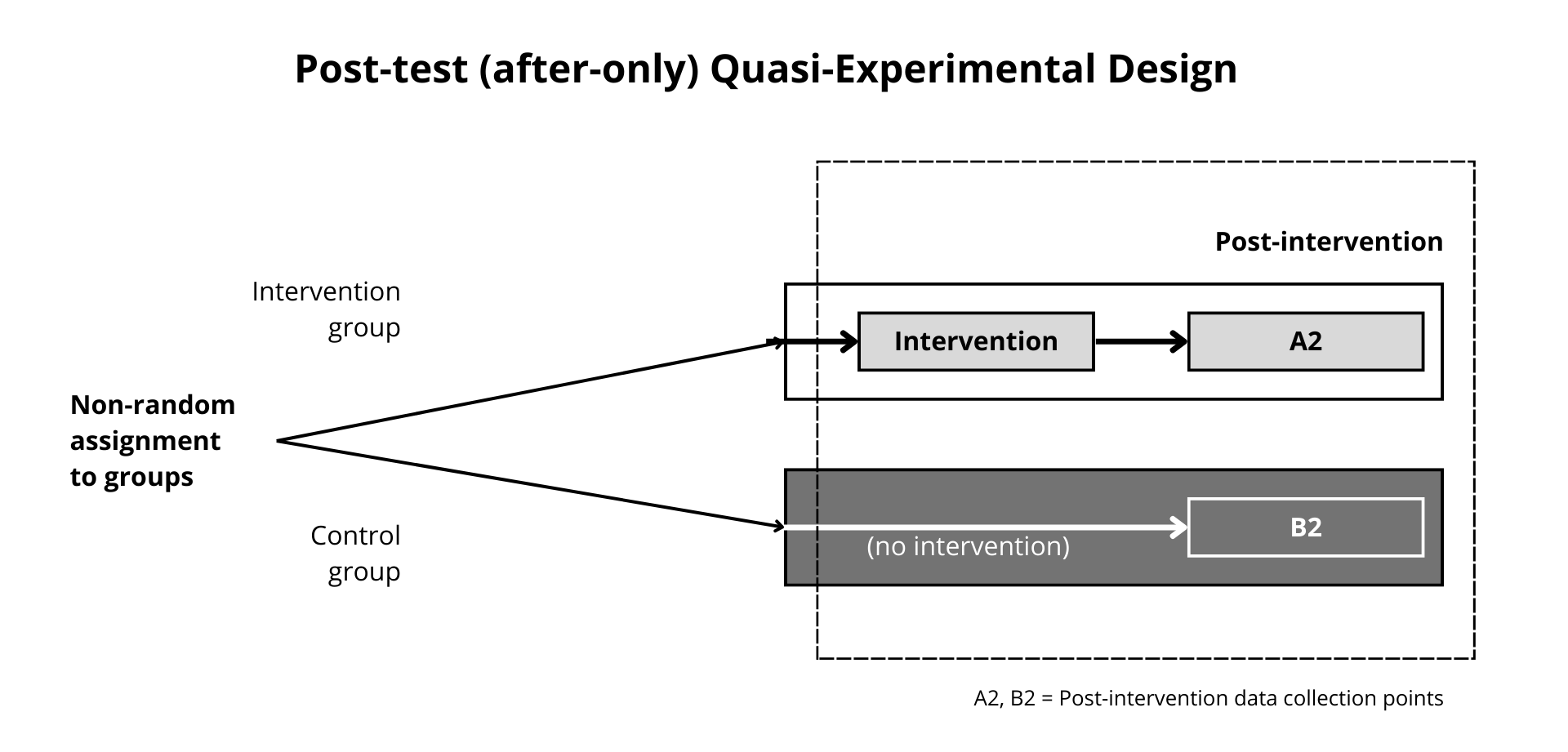
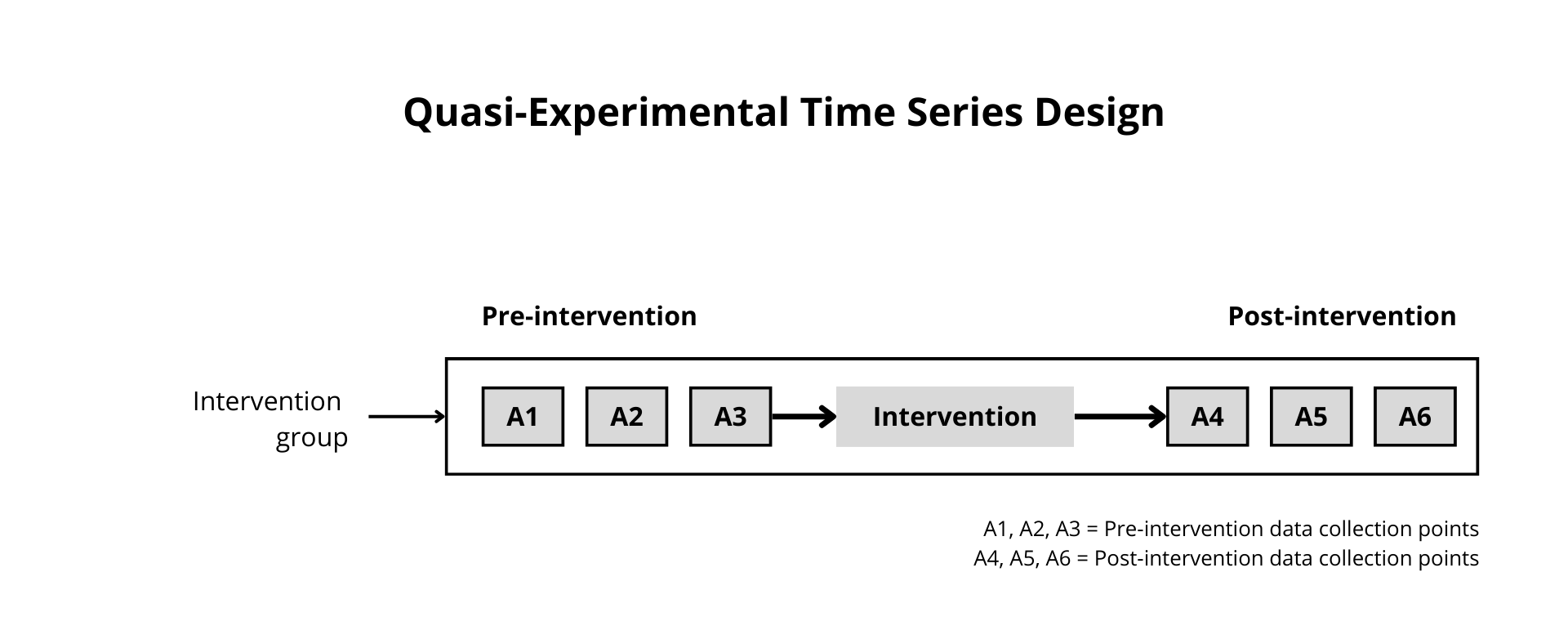
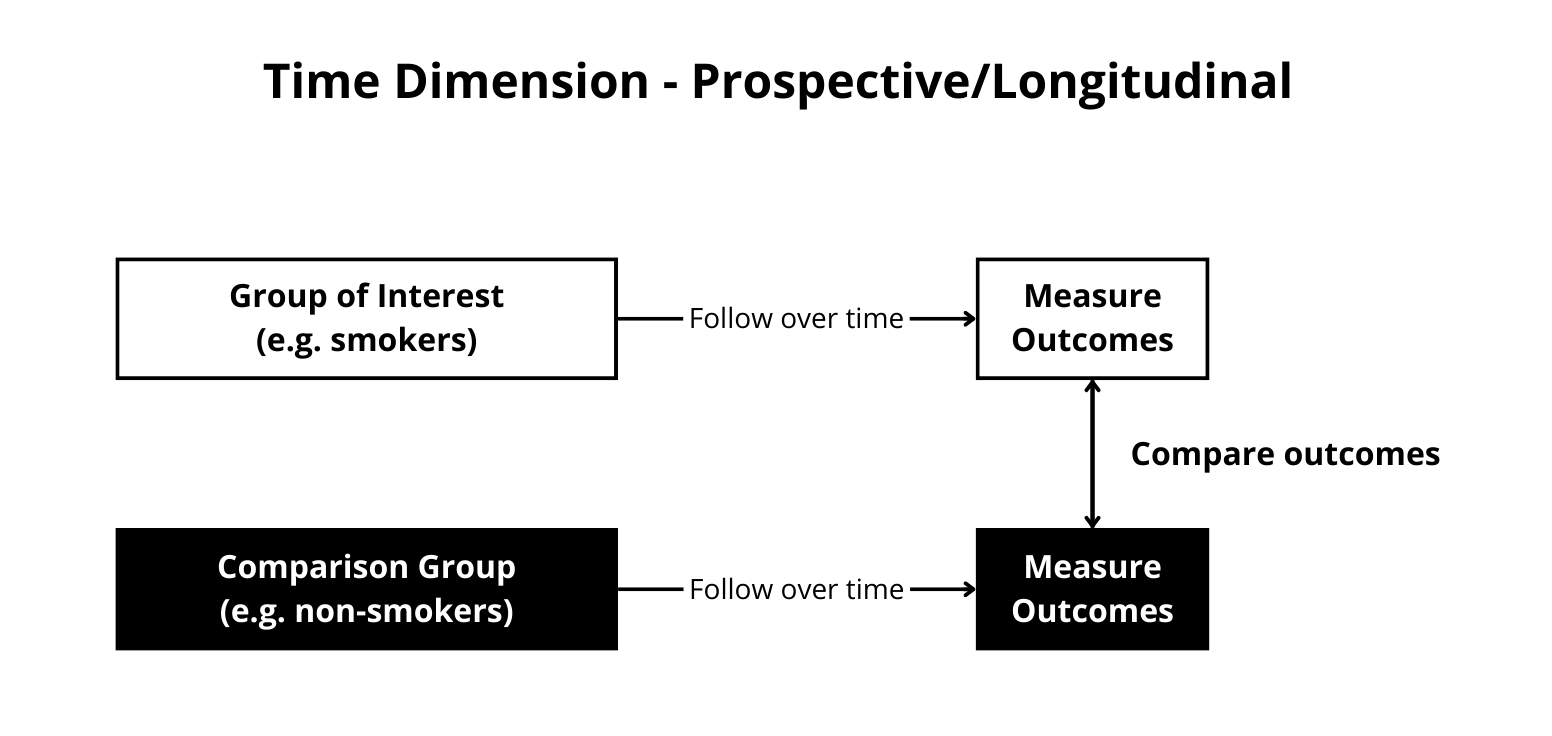
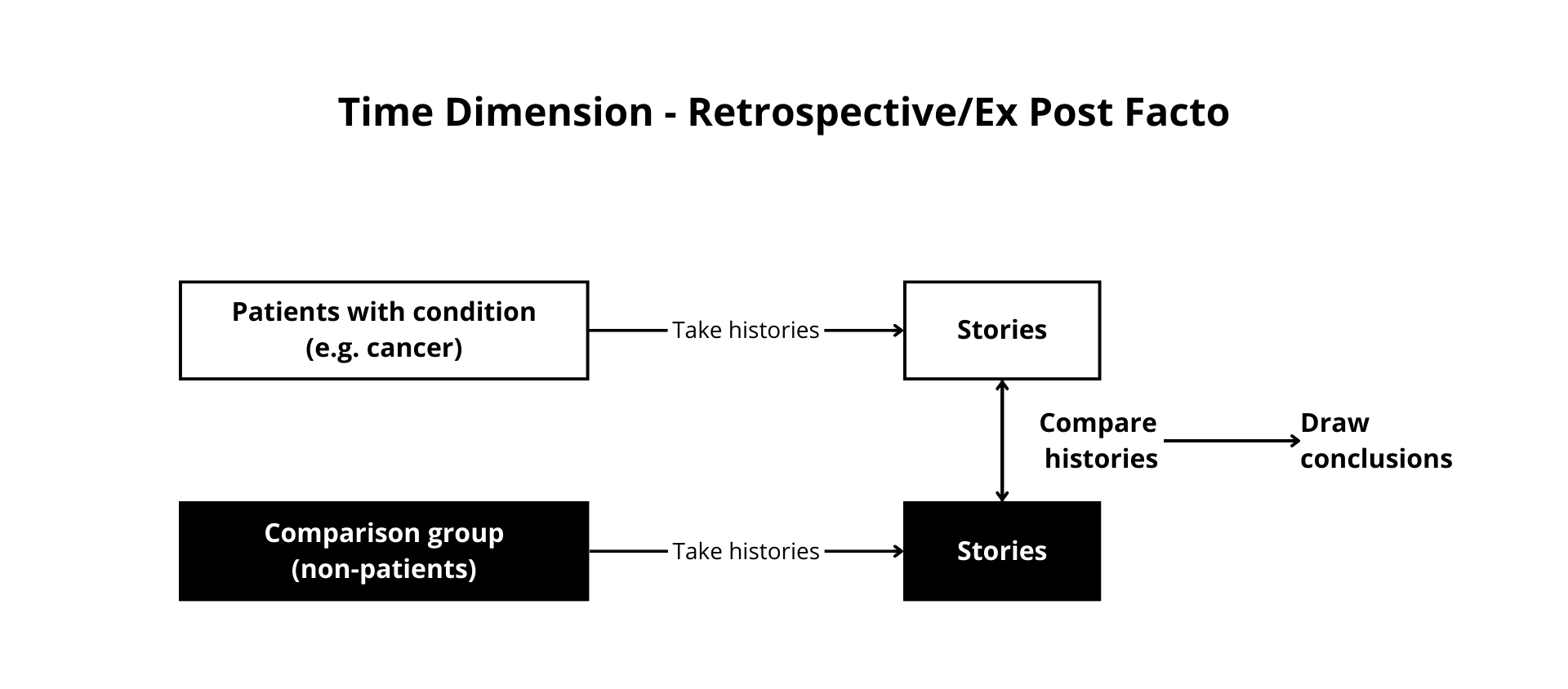
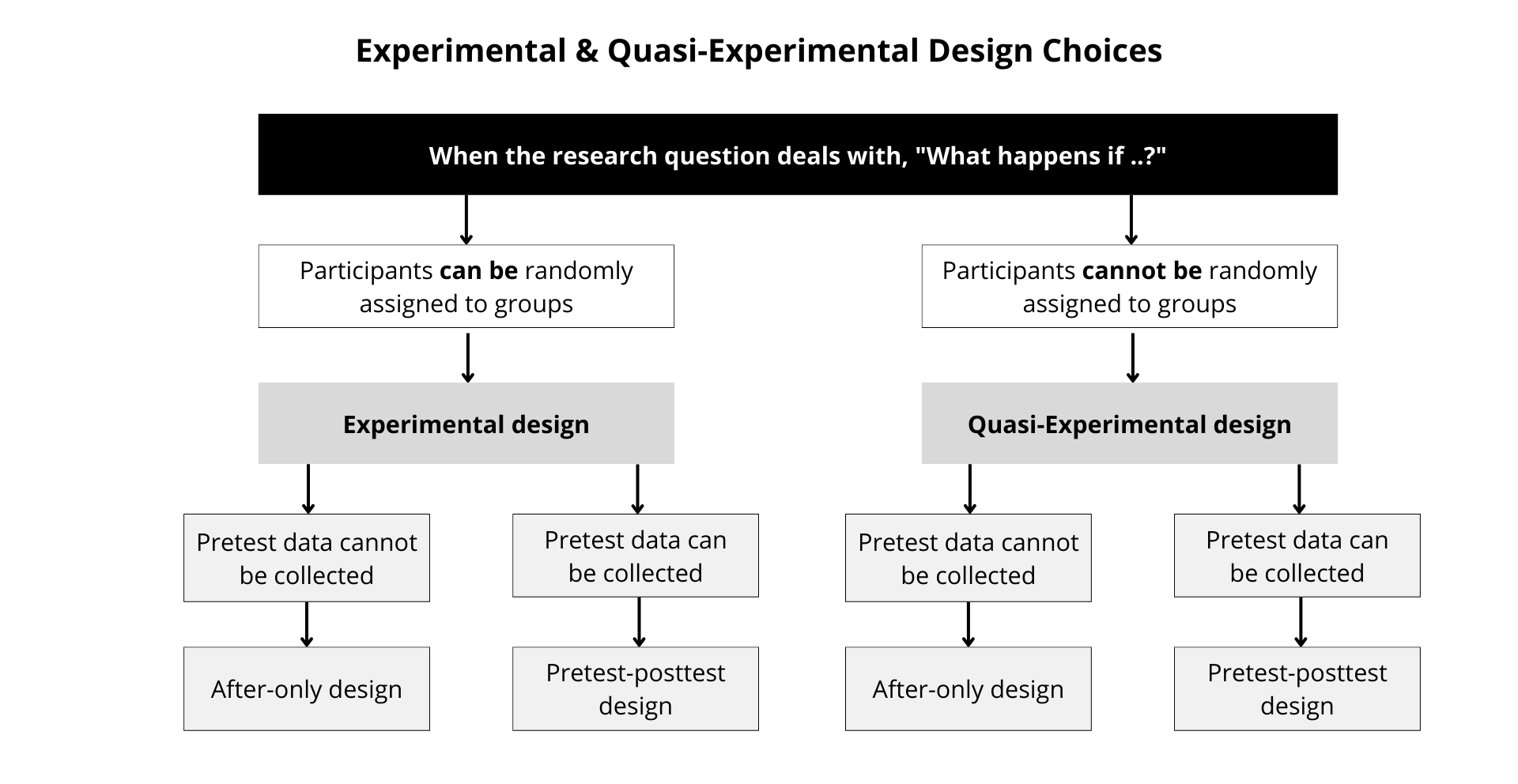
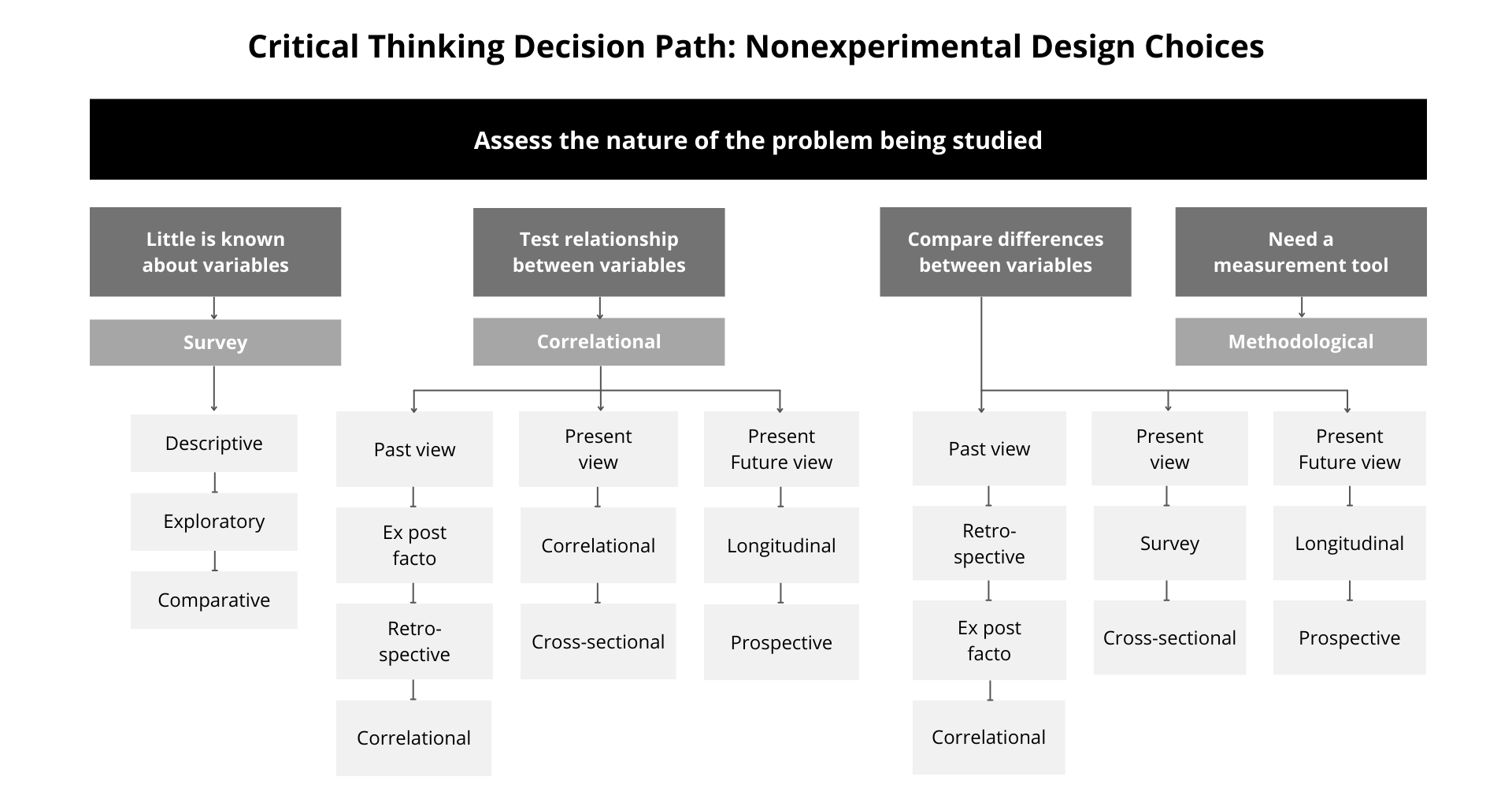
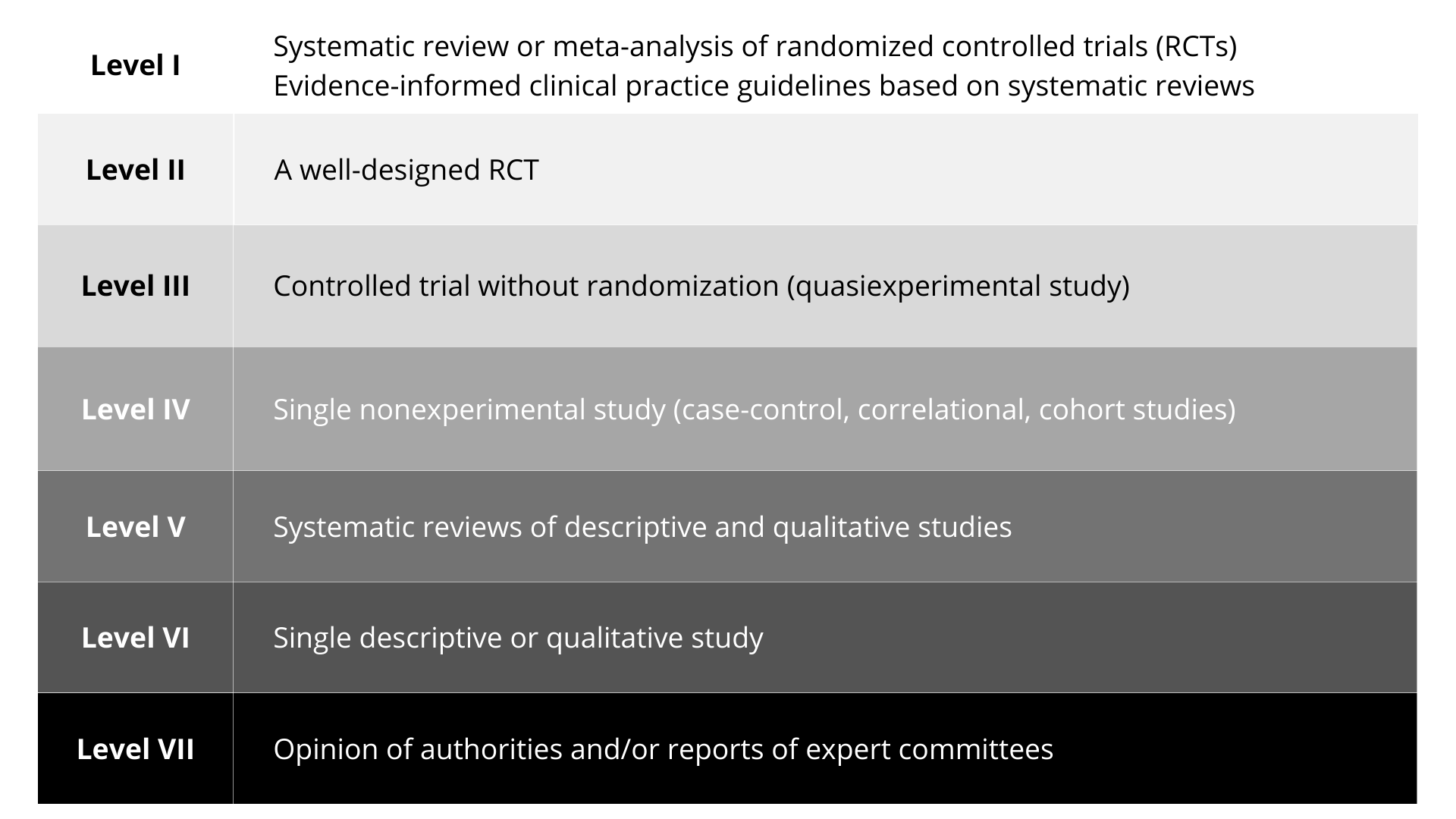 Figure 12.
Figure 12.